Effects of Fermented Milk with Live Bifidobacterium lactis Y6 on Intestinal Health in People with Digestive Dysfunction
-
摘要: 为了探究饮用乳双歧杆菌Y6活菌型发酵乳对消化功能障碍人群肠道健康的影响,本文进行了一项膳食干预试验。选择具有消化功能问题的受试者,每天饮用200 mL Y6活菌型发酵乳,持续4周。分别在干预前后进行临床评分,同时利用Illumina PE300测序平台,对受试者粪便微生物的16S rDNA PCR产物片段进行高通量测序,使用气相色谱测定了粪便中短链脂肪酸(SCFAs)的含量。结果表明,饮用Y6活菌型发酵乳对消化功能障碍导致的临床症状有极显著的改善作用(P<0.01);高通量测序结果显示,Y6活菌型发酵乳的干预对人体肠道微生物群的组成影响显著,但对物种多样性和丰富度没有明显改变,其中有益菌Akkermansia、Collinsella和Erysipelotrichacee_UCG_003属的相对丰度增加,有害菌Lachnoclostridium属的相对丰富度减少;肠道内SCFAs(乙酸、丙酸和丁酸)含量显著增加(P<0.05)。乳双歧杆菌Y6活菌型发酵乳的干预可以显著改善消化功能障碍人群的肠道健康,为Y6菌株的应用提供理论依据。
-
关键词:
- 乳双歧杆菌Y6 /
- 活菌型发酵乳 /
- 消化功能障碍 /
- 短链脂肪酸(SCFAs) /
- 肠道健康
Abstract: To investigate the effects of administering fermented milk with live Bifidobacterium lactis Y6 on intestinal health in people with digestive dysfunction, a dietary intervention experiment was conducted. Volunteers with digestive problems were selected to consume 200 mL Y6 fermented milk daily for 4 weeks. Clinical scores were performed before and after the intervention, the Illumina PE300 sequencing platform was used to perform high-throughput sequencing of 16S rDNA PCR product fragments of fecal microorganisms in volunteers, the content of short-chain fatty acids (SCFAs) in feces was determined using gas chromatography. The results showed that drinking Y6 fermented milk had a significant improvement on clinical symptoms caused by digestive dysfunction (P<0.01). High-throughput sequencing results showed that drinking Y6 fermented milk had significant effect on the composition of gut microbiota rather than the diversity and richness. The relative abundance of beneficial bacteria Akkermansia, Collinse and Erysipelotrichaceae_UCG_003 genera increased while the harmful bacteria Lachnoclostridium genera reduced. The contents of intestinal SCFAs (acetic acid, propionic acid and butyric acid) increased significantly (P<0.05). The intervention of fermented milk with live Bifidobacterium lactis Y6 can significantly improve the intestinal health of people with digestive dysfunction, providing a theoretical basis for the application of Y6 strains. -
人体肠道中包含着数以万计、种类丰富的微生物,它们与宿主间存在着相互依存、相互制约的关系。肠道菌群失衡会对宿主健康产生不良影响,导致一些肠道疾病的发生。较为常见的肠道疾病包括功能性消化不良(functional dyspepsia,FD)和肠易激综合征(irritable bowel syndrome,IBS)。前者的主要症状表现为上腹部疼痛或有烧灼感、餐后饱胀感或早饱等[1]。后者的症状表现为与大便形式或频率变化相关的腹痛[2]。此外,神经及心理不适、无明显肠功能障碍但伴随疼痛感增加的健康损害也是IBS多种亚型的重要典型特征[3],且IBS常与FD并存伴发[4]。患有FD和IBS会对人们的生活和工作产生不利影响[5−6],统计显示全球范围内患FD和IBS的人群占比分别约为7%~45%和11%(成年人)[7−8],人群规模相对较大。肠道健康问题备受科学研究者的关注,已成为健康医疗等领域的研究热点[9−10]。
益生菌具有改善肠道健康的功能[11],可通过调节肠道菌群,产生抗菌物质,修复和维护肠道粘膜屏障,激活机体免疫等方式来缓解IBS的临床症状[12]。研究表明摄入长双歧杆菌可以改善IBS的临床症状同时提高患者的生活质量[13]。Christopher等[14]分别利用嗜酸乳杆菌和乳双歧杆菌对IBS患者进行6周干预,结果表明2个益生菌组的IBS症状严重程度评分均显著下降(包括腹痛、腹胀、排便习惯和生活质量相关评分),且2个益生菌组的粪便性状均趋向正常化。有研究者发现,限制可发酵碳水化合物的摄入和补充益生元可以减轻一些IBS患者的症状,这可能跟饮食对肠道菌群的调节有关[15−16]。在FD治疗方面,Shahram等[17]、Zhang等[18]和Igarashi等[19]研究发现益生菌可以通过改变肠胃微生物组成从而改善整体消化不良的情况。Xue等[20]在FD大鼠模型中发现SCFAs浓度异常是FD的诱因之一。研究表明,肠道微生物群及其代谢产物SCFAs可能导致IBS的发病[21]。SCFAs可以维护肠道屏障、参与机体的代谢及免疫调节,维持中枢神经系统的发育和稳态等[22−23],能够通过微生物-肠-脑轴,在神经内分泌系统中参与疼痛、焦虑和压力的传递。因此,深入研究SCFAs在肠道健康中发挥的作用很有意义。
活菌型发酵乳是活性益生菌摄入的优良载体,能相对稳定地保持益生菌的活性[11]。当前研究活菌型发酵乳通过膳食干预影响人体肠道健康的文献并不多。本文通过膳食干预试验,探究了乳双歧杆菌Y6的活菌型发酵乳对人体肠胃不适症状的改善情况,同时对干预后的人体肠道菌群和肠道内SCFAs的含量进行了测定评估。本研究为乳双歧杆菌Y6的产品开发和应用研究提供理论依据。
1. 材料与方法
1.1 材料与仪器
乳双歧杆菌Y6(保藏号:CGMCC NO.15026) 广东益可维生物技术有限公司提供;生牛乳 广东燕塘乳业股份有限公司提供;发酵剂 美国丹尼斯克公司;含乳双歧杆菌Y6活菌型发酵乳,乳双歧杆菌Y6活菌数≥2×108 CFU/mL,200 mL/瓶 广东燕塘乳业股份有限公司提供;硫酸、乙醚 分析纯,国药集团化学试剂有限公司;0.22 μm滤膜 美国Millipore公司;乙酸、丙酸、丁酸均为色谱级,纯度大于99.0% 美国 Sigma 公司。
7890气相色谱分析仪 美国安捷伦公司;5424R高速冷冻离心机 德国Eppendorf公司;Vortex-5漩涡混匀仪 上海沪析公司;FA2104S电子天平 上海恒平公司;BCD-181MLC冷藏冷冻箱 合肥美菱公司;MDF-86V338医用低温保存箱 安徽中科都菱公司;巴氏杀菌机 利乐公司。
1.2 实验方法
1.2.1 样品制备
含乳双歧杆菌Y6活菌型发酵乳的制备:生牛乳85 ℃、15 s巴氏杀菌处理后冷却至40~45 ℃接种发酵剂与乳双歧杆菌Y6,灌装后拉入43 ℃温房发酵至终点酸度为70吉尔涅尔度后进行冷却后熟,成品2~8 ℃冷藏保存。
吉尔涅尔度的测定参照《GB 5009.239-2016 食品安全国家标准 食品酸度的测定》方法测定样品滴定酸度值,每个样品测定3次取其均值。
1.2.2 受试者选择及试验设计
1.2.2.1 纳入标准
选择功能性消化不良,伴有长期胃肠不适,主诉食欲不振、早饱、气多,胃肠胀满,呕吐,不明原因慢性腹泻或大便秘结等自愿受试者。
1.2.2.2 排除标准
急性腹泻者;严重器质性病变引起的消化不良者;体质虚弱无法接受试验者;合并有心血管、肝、肾和造血系统等严重全身性疾病患者;未按要求服用受试样品,无法判断饮用结果者;乳糖不耐受或牛奶过敏者;在受试前1周内服用过抗生素,在受试前1个月内服用过泻药抗腹泻药物,在受试前2个月内参加过其他研究项目,受试前2周内连续服用过益生菌及其产品者。
1.2.2.3 受试对象
试验共招募30人,男女不限,年龄20~60岁,试验前均签署知情同意书。
1.2.2.4 食用方法
膳食干预实验共35 d,包括7 d排空期和28 d饮用期。乳双歧杆菌Y6活菌型发酵乳样品置于4 ℃冰箱存放,受试者在饮用期内每日午饭或晚饭后1 h以内饮用,1瓶/日。分别在排空期结束和饮用期结束时收集粪便样本,并由医生进行临床症状评分[24](如表1所示),其中无明显症状者可打0分。在整个实验过程中,受试者不可食用任何发酵食品(包括泡菜、腐乳、酸奶、奶酪、活性乳酸菌饮料等),但可饮用牛奶。
1.2.3 肠道菌群测定
粪便收集后快速密封并于-81 ℃速冻保存,使用干冰寄送至上海美吉生物,按照粪便DNA提取试剂盒说明书对样本进行总DNA的提取,肠道菌群多样性测序利用Illumina PE300测序平台,对16S rDNA PCR产物片段进行高通量测序,测序工作由上海美吉生物医药科技有限公司完成,基于I-Sanger云平台(https://www.i-sanger.com/)进行生物信息学及数据统计分析。
生物信息分析方法:依据overlap关系对PE reads进行拼接, 同时对序列质量质控和过滤,各样本进行OTU聚类分析和物种分类学分析,基于OTU进行多样性指数分析。在各分类水平上进行群落结构的统计分析。并对多样本的群落组成信息进行分析和差异显著性检验等。
1.2.4 短链脂肪酸(SCFAs)含量测定
标准品配制:取适量乙酸、丙酸、丁酸标准品,用乙醚配制成0.1、0.5、2、5、10、50、100、250、500 μg/mL的混合标准浓度梯度。母液及工作标准溶液均保存于0 ℃。
粪便样品处理根据张炎等[25]方法加以改进,称取1.0 g粪便置于15 mL离心管,加入10 mL ddH2O和适量玻璃珠振荡分散10 min,4000×g离心15 min,取上清液,过0.22 μm滤膜收集滤液,取2 mL过滤液于离心管,加入50%的硫酸溶液0.2 mL,乙醚2.0 mL,振荡10 min后4000×g离心15 min,10 ℃静置30 min,取上层乙醚溶液进行气相色谱分析,将样品与标品谱图对比进行定性,采用外标法绘制标准曲线进行定量分析。
色谱条件:FID氢火焰离子检测器;60~80目(酸洗、硅烷化)不锈钢柱(25 m×0.320 mm)色谱柱,涂渍填充10% FFAD和1% H3PO4;升温程序:80 ℃保持1 min,以10 ℃/min升至140 ℃,保持5 min,以25 ℃/min升至240 ℃,保持4 min;载气(N2)压力260 kPa;氮气流速为40 mL/min,氢气流速为30 mL/min,空气流速为400 mL/min;进样量1 μL;检测温度280 ℃。N2000色谱工作站收集信号并检测。
1.3 数据处理
利用SPSS 21.0软件对结果进行统计分析,各项指标结果以“平均值±标准差”(mean±SD)表示。自身对照资料采用配对t检验,P<0.05视为显著性差异,具有统计学意义。
2. 结果与分析
2.1 受试者基本情况
受试者30人,脱落5人,有效受试样本25人,基本情况(年龄、性别、BMI等)见表2。
表 2 受试者饮用前基本情况Table 2. Basic information of subjects before ingesting项目 数值 性别 男 6 女 19 年龄(岁) 47.8±8.71 BMI指数 23.6±3.20 2.2 临床症状评分变化
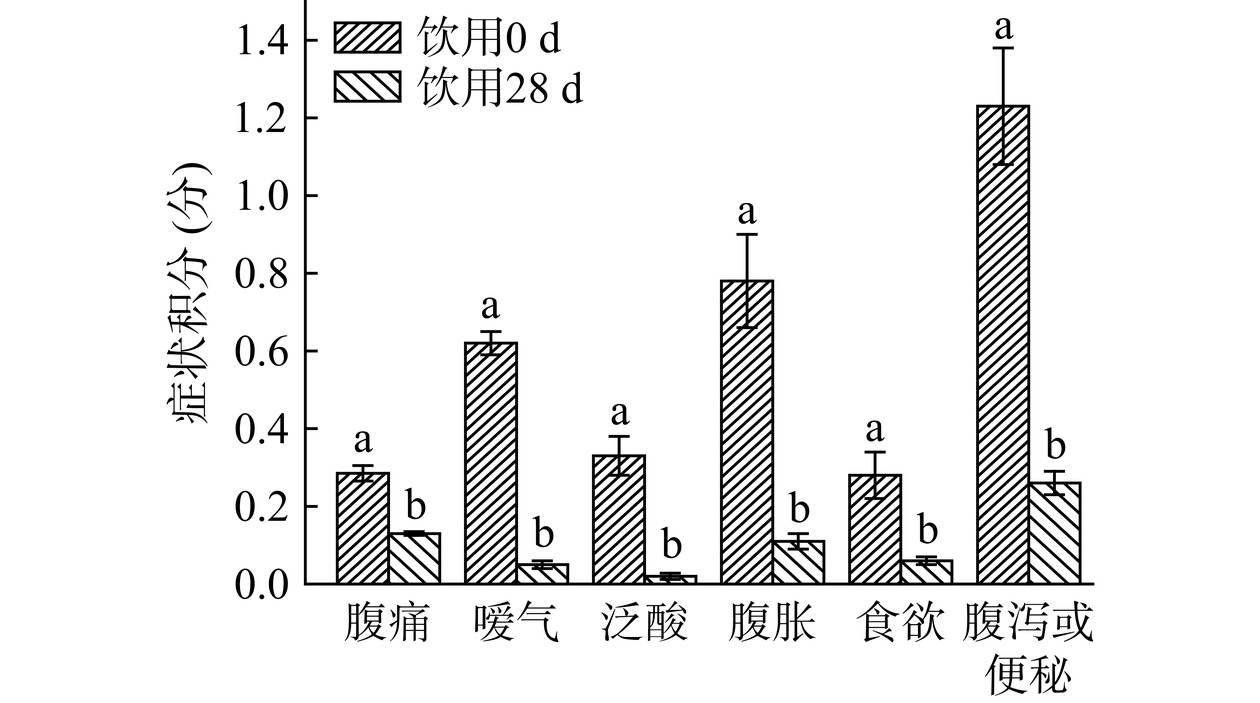
受试者饮用前后的临床症状评分如图1所示。饮用28 d,受试者嗳气、泛酸、食欲不振症状完全消失(症状积分<0.1),腹痛、腹胀、腹泻或便秘等其他症状评分极显著降低(P<0.01),说明乳双歧杆菌Y6活菌型发酵乳对功能性消化不良人群的临床症状有显著的改善效果。
![]() 图 1 人群饮用前后临床症状积分的变化注:采用配对t检验,P<0.05为显著性差异,采用LSD字母标记法;图8同。Figure 1. Change in clinical symptom score before and after ingesting
图 1 人群饮用前后临床症状积分的变化注:采用配对t检验,P<0.05为显著性差异,采用LSD字母标记法;图8同。Figure 1. Change in clinical symptom score before and after ingesting2.3 肠道菌群的变化
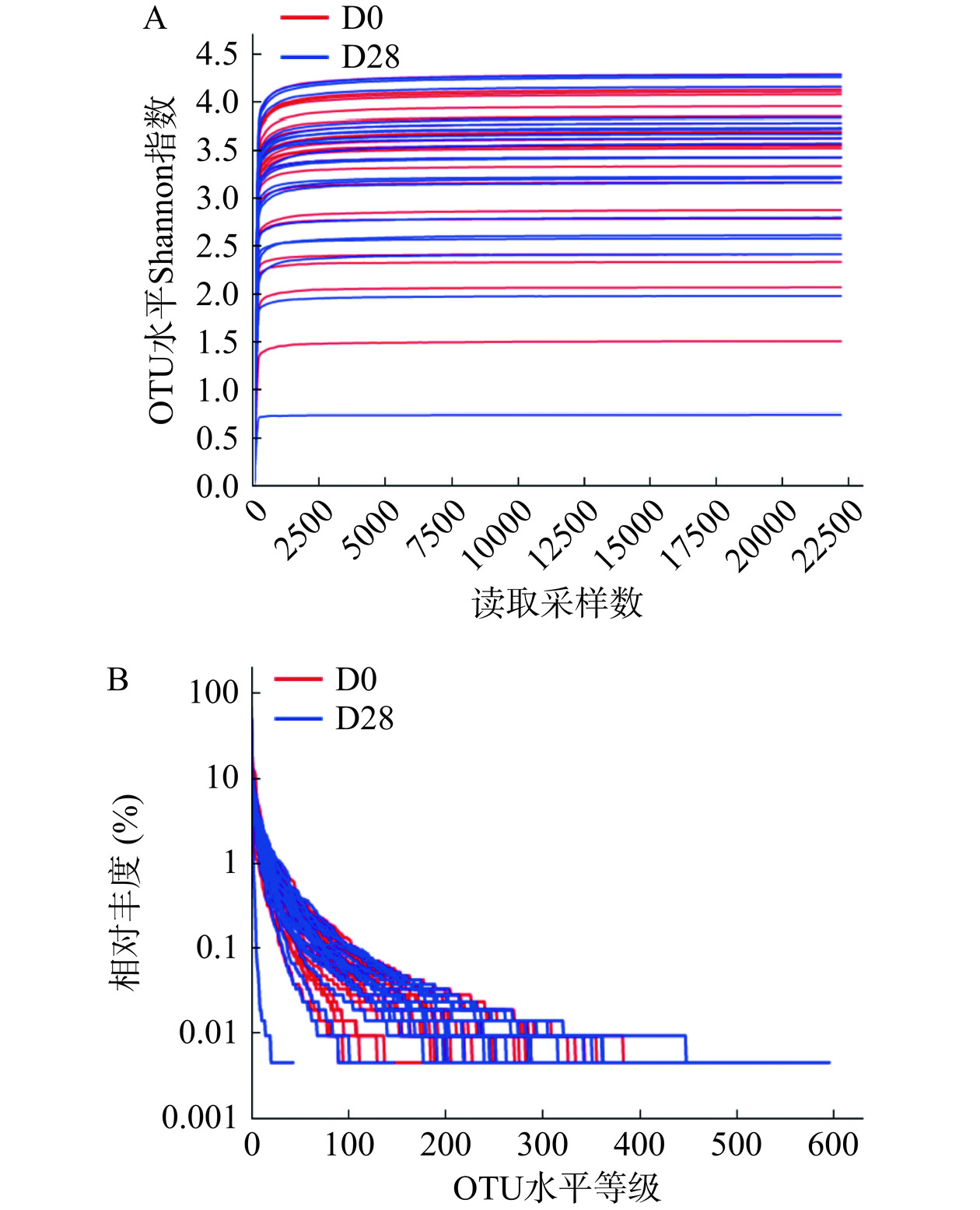
2.3.1 粪便样本的稀释曲线与Rank-Abundance曲线
由图2可知,对50个样本进行测序,基于97%相似度的分类水平,共注释到2019个OTUs,测序深度可以覆盖样品中的绝大部分微生物种类。Rank-Abundance曲线显示相对丰度高于1%的细菌种类在各样本中均较少,随样本中物种组成丰富度的增加,曲线逐渐平坦,物种组成的均匀度逐渐提高。当样本中相对丰度低于0.01%时,曲线趋于水平,不同样本在不同OTU丰度达到平台期。
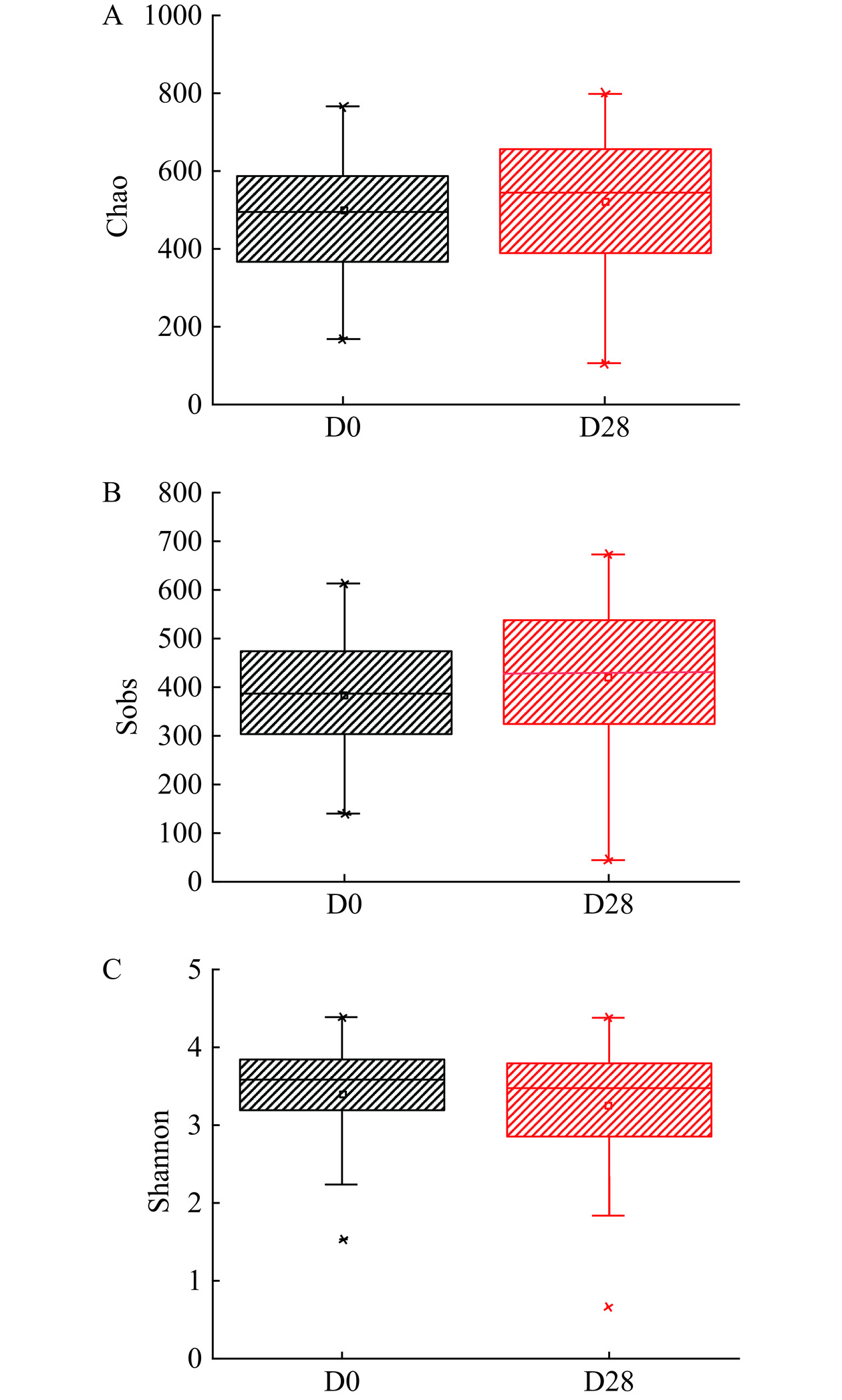
2.3.2 多样性指数
如图3所示,饮用后与饮用前相比,Chao指数与Sobs有所增加但不显著(P>0.05),Shannon指数无显著性差异(P>0.05),说明饮用前后肠道菌群的物种多样性和丰富度没有显著改变。
2.3.3 物种组成分析
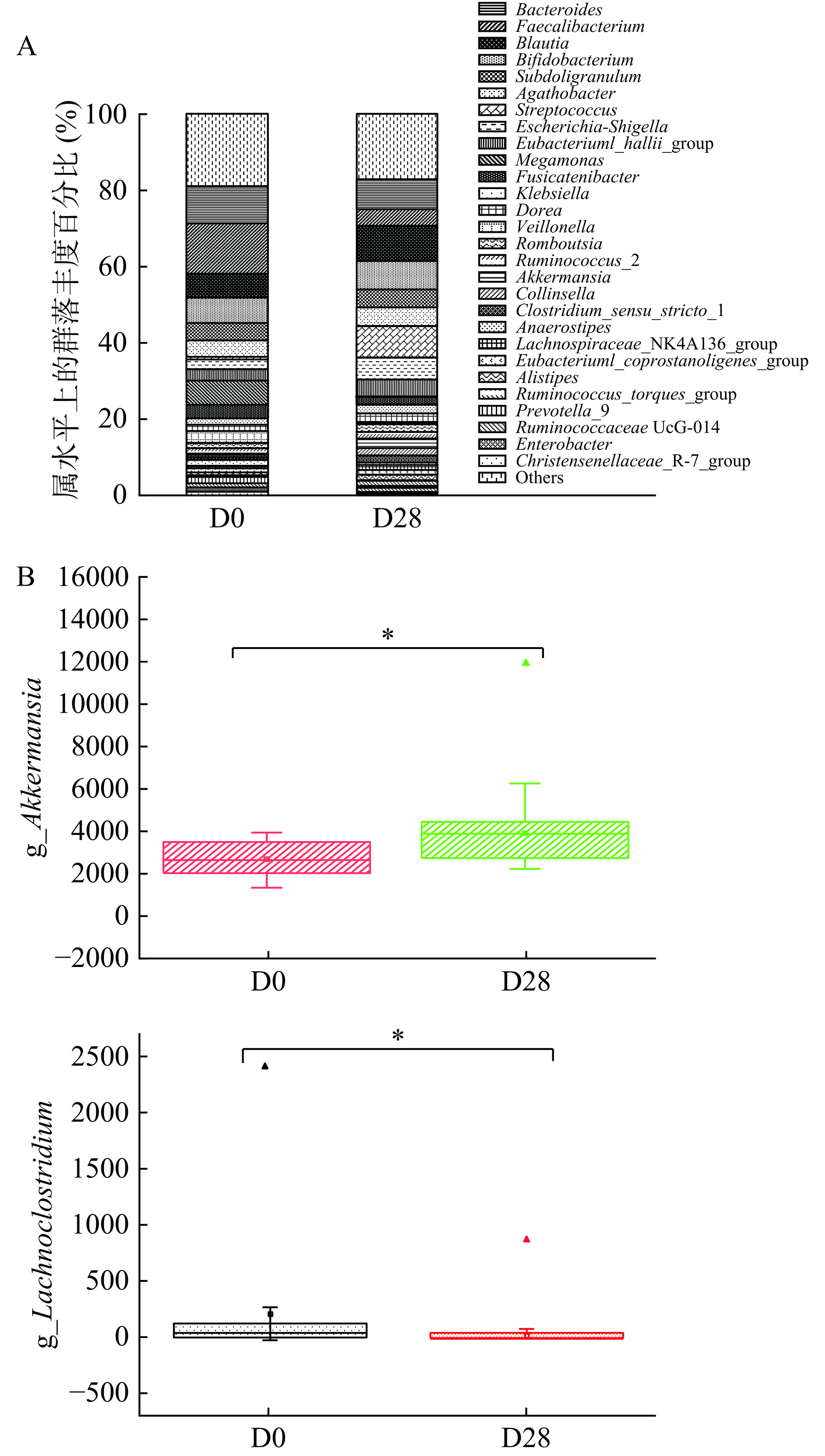
在门水平,如图4所示,干预前后肠道菌群中的优势菌门均为拟杆菌门(Bacteroidetes)、变形菌门(Proteobacteria)、放线菌门(Actinobacteria)等。与饮用前相比,饮用期第28 d,厚壁菌门(Firmicutes)、拟杆菌门、放线菌门相对丰度增加,变形菌门、疣微菌门(Verrucomicrobia)相对丰度减少。IBS是一种常见的功能性肠性疾病,具有复杂的病理生理学机制,可能跟胃肠道的异常运动,肠道的过敏反应,肠道微生物群的改变,胃肠道感染后的低度炎症等因素有关[26]。有文献报道,IBS患者肠道内的厚壁菌门数量较多,拟杆菌门和放线菌门数量相对较少[27−28]。裘建明等[29]对肠道微生物菌群进行研究,发现厚壁菌门的部分菌属能够降解肠道黏蛋白,改变肠道黏膜的通透性,导致炎性反应的发生。拟杆菌门的部分菌属能够辅助刺激T细胞介导从而出现肠道黏膜的炎性反应。以上结果表明,肠道中厚壁菌门和拟杆菌门数量的降低,能够在一定程度减少肠道炎性反应的发生,从而在一定程度上缓解IBS的症状,与本文的干预结果相似。
在属水平,对相对丰度大于1%的菌属进行分析,结果如图5,与饮用前相比,饮用期第28 d,显著增加的有益菌属为阿克曼氏菌(Akkermansia),有害菌属Lachnoclostridium的相对丰度显著降低(P<0.05)。Akkermansia属于疣微菌门,可以改善肠道屏障功能,增加黏液层厚度,降解黏蛋白,具有免疫调节作用,能够缓解结肠炎的部分症状[30]。Lachnoclostridium菌属于厚壁菌门,其相对丰度在不同的健康状态下存在差异,在溃疡性结肠炎和肠易激综合征患者的肠道菌群中,Lachnoclostridium属的含量较高[31]。综合以上结果可以看出,饮用乳双歧杆菌Y6活菌型发酵乳可以改变肠道菌群属的水平,使其中的有益菌Akkermansia增加,有害菌Lachnoclostridium降低,从而改善肠道不适症状。
2.3.4 干预人群肠道菌群群体间多样性分析
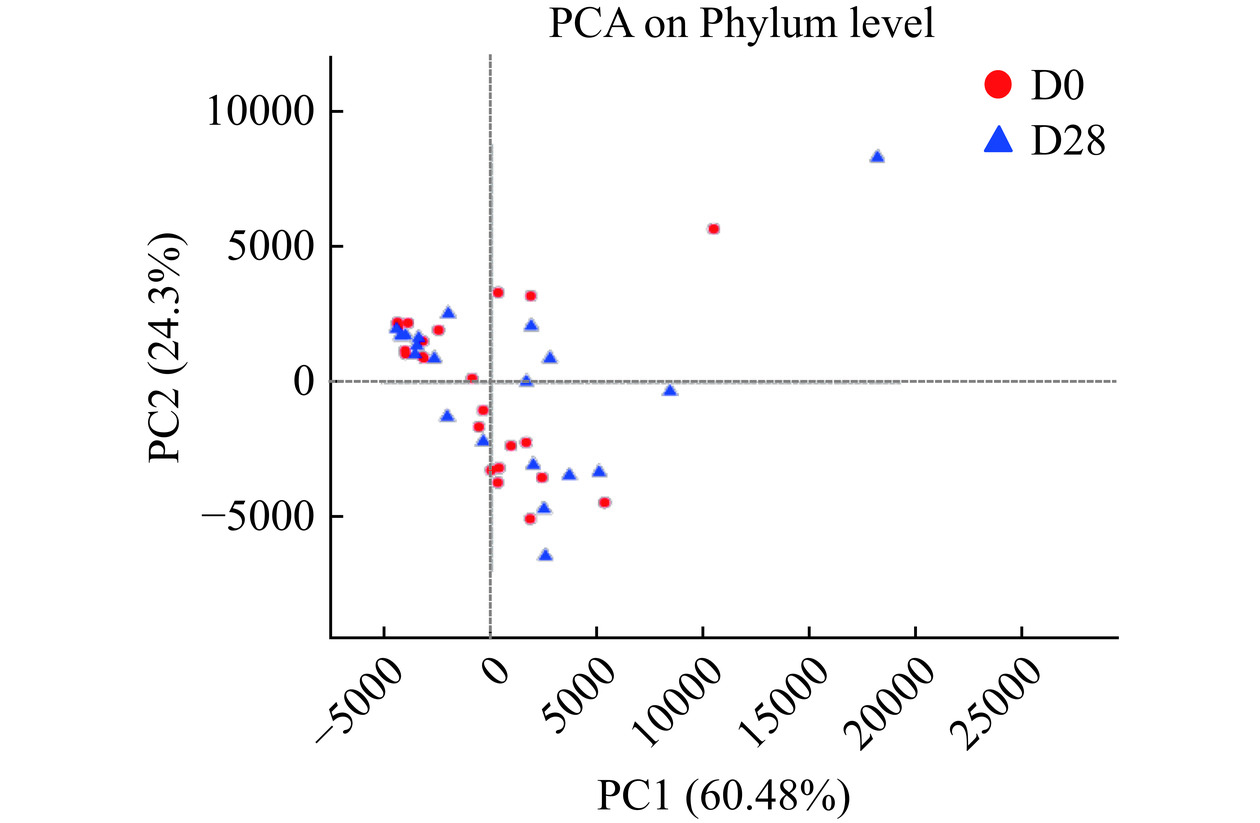
饮用前后人群肠道菌群结构的PCA分析如图6所示,PC1为第一主坐标,对整体菌群的代表性为60.48%,PC2为第二主坐标,对整体菌群的代表性为24.3%,饮用前后,群落整体位于右侧,各组之间未分开,说明各组菌群组成未发生显著改变。
2.3.5 物种差异分析
饮用前后人群肠道菌群LEfSe(wilcox test,P<0.05,LDA=3.0)多级物种差异判别分析如图7所示,在饮用前,受试者肠道中主要富集的菌为Negativicutes,Selenomonadales,瘤胃球菌科(Ruminococcaceae),粪杆菌属(Faecalibacterium)和Lachnoclostridium。在饮用乳双歧杆菌Y6活菌型发酵乳28 d后,受试者肠道中主要富集的菌为芽孢杆菌纲(Bacilli),丹毒丝菌纲(Erysipelotrichia),乳杆菌目(Lactobacillales),丹毒丝菌目(Erysipelotrichales),链球菌科(Streptococcaceae),红蝽菌科(Coriobacteriaceae),丹毒丝菌科(Erysipelotrichaceae),Eggerthellaceae,链球菌纲(Streptococcus),柯林斯菌属(Collinsella)和Erysipelotrichaceae_UCG_003。
Bacilli在肠道中处于正常水平时是有益菌,能够产生多种酶类,促进营养物质的消化吸收。Erysipelotrichia、Erysipelotrichales当前在人类肠道微生物群中的重要性仍未得到明确研究[32]。而Lactobacillales是肠道中的重要益生菌,其代谢产物可以有效降低肠道内pH,促进肠蠕动,调节肠道健康[33]。Streptococcaceae、Streptococcus是肠道中多样性的菌属[34],正常情况下对人体无害。Coriobacteriaceae 可以产生短链脂肪酸从而维持肠道健康稳定[35]。
Eggerthellacea在肠道中能够分解鞣花酸产生尿石素,增加连接蛋白的紧密水平,减少肠道炎症,从而改善肠道健康[36]。Collinsella属于放线菌门,与高水平马尿酸有关,马尿酸是肠道衍生的代谢产物,可作为健康的表征,Erysipelotrichaceae_UCG_003属能够将复杂的碳水化合物消化并代谢为SCFAs[34]。由以上结果可知,饮用Y6活菌型发酵乳能够增加受试者肠道中的Lactobacillales 、Coriobacteriaceae 、Collinsella和Erysipelotrichaceae_UCG_003,可以通过在肠道中产生有益代谢产物从而改善肠道健康。
已有研究表明,食用益生菌产品主要通过调节肠道菌群,改善不正常的肠道微生物结构来缓解受试者的肠道不适症状,使其恢复正常的生理机能[37]。Harata等[38]使用LGG-TMC0356发酵乳对相关人群进行干预,结束时发现Collinsella和Lactobacillus等菌属的丰度显著增加,有效调节受试者的肠道菌群,从而改善相关不适症状。臧凯丽等[39]研究表明,益生菌干预能够调控肠道菌群多样性,同时提高肠道菌群的相对丰度,可以增加肠道不健康人群Lactobacillus 和Collinsella的丰度,且能保持一段时间的稳定。孙二娜等[40]发现食用乳酸菌发酵饮料可以显著增加肠道内的有益菌Lactobacillus和Muribaculaceae菌属的丰度,从而缓解消化不良表现出的嗳气、泛酸、腹胀、腹痛等症状。Yang等[41]用植物乳杆菌CCFM1143治疗慢性腹泻,结果表明植物乳杆菌CCFM1143治疗组显著降低了慢性腹泻患者肠道内的Lachnoclostridium和Lachnospira的相对丰度,能够减轻患者的临床症状,改善生活质量。
2.4 人群粪便SCFAs含量变化
受试者饮用前后粪便样本SCFAs的含量变化如图8所示,饮用后第28 d与饮用第0 d相比,粪便中乙酸、丙酸、丁酸含量显著增加(P<0.05),表明饮用乳双歧杆菌Y6活菌型发酵乳28 d可以增加肠道内SCFAs含量。SCFAs对肠黏膜屏障的维护、肠易激综合征的调节、免疫调节、抗癌等有积极效果[42]。张浩[43]研究发现SCFAs在IBS等患者粪便中显著减少,其中丁酸减少的最为明显。在肠道多种微生物混合发酵的体系中,糖分解代谢的主要最终产物是SCFAs、乙酸盐、丙酸盐和丁酸盐,它们占结肠总SCFAs的85%~95%[44]。大多数产丁酸的细菌不能直接利用某些益生元物质,但乳酸菌可以将这些益生元完全分解,产生乙酸和乳酸等代谢终产物。产丁酸细菌能够进一步将这两种类型的产物作为次生基质,发酵产生丁酸[45−46]。因此乳酸菌与产丁酸细菌的交互共生关系也是受试者在饮用Y6活菌型发酵乳后丁酸含量增加的可能原因之一。
3. 结论
本试验研究表明,受试者在饮用28 d乳双歧杆菌Y6活菌型发酵乳后,临床症状评分结果表明嗳气、泛酸、食欲不振等症状完全消失,腹痛、腹胀、腹泻或便秘等其他症状评分极显著降低(P<0.01),乳双歧杆菌Y6活菌型发酵乳对功能性消化不良人群的临床症状有显著的改善效果。另外,乳双歧杆菌Y6活菌型发酵乳可调节肠道菌群,与饮用前相比,人群肠道的厚壁菌门、拟杆菌门、放线菌门相对丰度增加,变形菌门、疣微菌门相对丰度减少。属水平上,Akkermansia菌属显著增加,Lachnoclostridium菌属的相对丰度显著降低(P<0.05);物种差异分析表明Y6活菌型发酵乳膳食干预后可以增加受试者肠道中Collinsella和Erysipelotrichaceae_UCG_003属;此外,随着肠道菌群的改变,肠道中的SCFAs含量增加。因此,饮用乳双歧杆菌Y6活菌型发酵乳有助于改善肠道微生态环境,调节肠道菌群,对促进肠道健康具有积极的作用。
-
图 1 人群饮用前后临床症状积分的变化
注:采用配对t检验,P<0.05为显著性差异,采用LSD字母标记法;图8同。
Figure 1. Change in clinical symptom score before and after ingesting
症状 轻(1分) 中(2分) 重(3分) 腹痛 持续时间短,不需服药 疼痛时间较长,每日超4 h,尚能忍受 疼痛较重,持续,需服药才能减轻 嗳气 间有发作 经常发作,引及两胁不适 频繁发作,引及两胁疼痛 泛酸 偶有吐酸 饮食不适即吐酸 频繁吐酸 腹胀 腹胀在短时间内较甚 腹胀较甚,在较长时间内不缓解 整日腹胀 食欲 食欲较差,饭量减少1/2以内 食欲差,饭量减少1/2~2/3 饭量减少2/3以上 腹泻或
便秘偶有腹泻或便秘 饮食不适即腹泻或便秘 频繁腹泻或便秘 表 2 受试者饮用前基本情况
Table 2 Basic information of subjects before ingesting
项目 数值 性别 男 6 女 19 年龄(岁) 47.8±8.71 BMI指数 23.6±3.20 -
[1] FORD A C, MAHADEVA S, CARBONE M F, et al. Functional dyspepsia[J]. The Lancet,2020,396(10263):1689−1702. doi: 10.1016/S0140-6736(20)30469-4
[2] FORD A C, SPERBER A D, CORSETTI M, et al. Irritable bowel syndrome[J]. The Lancet,2020,396(10263):1675−1688. doi: 10.1016/S0140-6736(20)31548-8
[3] BYALE A, LENNON R J, BYALE S, et al. High-dimensional clustering of 4000 irritable bowel syndrome patients reveals seven distinct disease subsets[J]. Clinical Gastroenterology and Hepatology, 2022, doi: 10.1016/j.cgh.2022.09.019.
[4] 庄莹, 林志辉. 27例腹泻型肠易激综合征患者食物不耐受与结肠黏膜肥大细胞、P物质含量的相关性[J]. 中华消化杂志,2016,36(2):91−95. [ZHUANG Y, LIN Z H. Association between food intolerance and colonic mucosal mast cells and substance P contents in 27 patients with diarrheal irritable bowel syndrome[J]. Chinese Journal of Digestion,2016,36(2):91−95.] doi: 10.3760/cma.j.issn.0254-1432.2016.02.004 ZHUANG Y, LIN Z H. Association between food intolerance and colonic mucosal mast cells and substance P contents in 27 patients with diarrheal irritable bowel syndrome[J]. Chinese Journal of Digestion, 2016, 36(2): 91−95. doi: 10.3760/cma.j.issn.0254-1432.2016.02.004
[5] 刘立芬, 李稳, 杨冬林, 等. 功能性消化不良与心理、生活事件及生活质量的关联性研究[J]. 国际精神病学杂志,2017,44(1):102−105. [LIU L F, LI W, YANG D L, et al. A study on the correlation between functional dyspepsia and psychological, life events and quality of life[J]. Journal of International Psychiatry,2017,44(1):102−105.] LIU L F, LI W, YANG D L, et al. A study on the correlation between functional dyspepsia and psychological, life events and quality of life[J]. Journal of International Psychiatry, 2017, 44(1): 102−105.
[6] GOODOORY V C, NG C E, BLACK C J, et al. Impact of rome IV irritable bowel syndrome on work and activities of daily living[J]. Alimentary Pharmacology & Therapeutics,2022,56(5):844−856.
[7] LEE H, JUNG H, HUH K C. Current status of functional dyspepsia in Korea[J]. The Korean Journal of Internal Medicine,2014,29(2):156. doi: 10.3904/kjim.2014.29.2.156
[8] 胡玥, 吕宾. 肠易激综合征的治疗进展[J]. 中国实用内科杂志,2020,40(2):105−110. [HU Y, LÜ B. Progress in the treatments of irritable bowel syndrome[J]. Chinese Journal of Practical Internal Medicine,2020,40(2):105−110.] HU Y, LÜ B. Progress in the treatments of irritable bowel syndrome[J]. Chinese Journal of Practical Internal Medicine, 2020, 40(2): 105−110.
[9] 中国微生态调节剂临床应用专家共识(2020版)[J]. 中国微生态学杂志, 2020, 32(8):953−965. [Expert consensus on clinical application of Chinese microecological regulators (2020 edition)[J]. Chinese Journal of Microecology, 2020, 32(8):953−965.] Expert consensus on clinical application of Chinese microecological regulators (2020 edition)[J]. Chinese Journal of Microecology, 2020, 32(8): 953−965.
[10] 中国食品科学技术学会益生菌分会. 益生菌的科学共识(2020年版)[J]. 中国食品学报,2020,20(5):303−307. [Probiotics branch of Chinese institute of food science and technology. Scientific consensus on probiotics (2020 editon)[J]. Chinese Institute of Food Science and Technology,2020,20(5):303−307.] Probiotics branch of Chinese institute of food science and technology. Scientific consensus on probiotics (2020 editon)[J]. Chinese Institute of Food Science and Technology, 2020, 20(5): 303−307.
[11] 伍鹏, 王娟, 王晶晶, 等. 基于仿生胃肠道模型的发酵乳中益生菌存活率评价[J]. 食品与发酵工业,2021,47(12):147−153. [WU P, WANG J, WANG J J, et al. Evaluation of probiotic survival rate in fermented milk based on a biomimetic gastrointestinal model[J]. Food and Fermentation Industries,2021,47(12):147−153.] WU P, WANG J, WANG J J, et al. Evaluation of probiotic survival rate in fermented milk based on a biomimetic gastrointestinal model[J]. Food and Fermentation Industries, 2021, 47(12): 147−153.
[12] 何佳璨, 周桂荣, 李欣欣, 等. 肠道菌群与肠易激综合征相关研究进展[J]. 中国微生态学杂志,2020,32(1):117−124. [HE J C, ZHOU G R, LI X X, et al. Progress in research on intestinal flora and irritable bowel syndrome[J]. Chinese Journal of Microecology,2020,32(1):117−124.] HE J C, ZHOU G R, LI X X, et al. Progress in research on intestinal flora and irritable bowel syndrome[J]. Chinese Journal of Microecology, 2020, 32(1): 117−124.
[13] JEAN-MARC S, FRANCK I. Effect of Bifidobacterium longum 35624 on disease severity and quality of life in patients with irritable bowel syndrome[J]. World Journal of Gastroenterology,2022,28(7):732−744. doi: 10.3748/wjg.v28.i7.732
[14] CHRISTOPHER J M, SHALINI S, GREGORY J L. Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABla-12 improve abdominal pain severity and symptomology in irritable bowel syndrome:Randomized controlled trial[J]. Nutrients,2020,12(2):363. doi: 10.3390/nu12020363
[15] MAGNUS S. Manipulating the gut microbiome as a treatment strategy for functional gastrointestinal disorders[J]. Gastroenterology,2018,155(4):960−962. doi: 10.1053/j.gastro.2018.09.008
[16] JOSE-WALTER H, MARIANELA M, CHAYSAVANH M, et al. Effects of prebiotics vs a diet low in fodmaps in patients with functional gut disorder[J]. Gastroenterology,2018,155(4):1004−1007. doi: 10.1053/j.gastro.2018.06.045
[17] SHAHRAM A, ABOLFAZL A, JAVAD H, et al. Systematic review with meta-analysis:Effects of probiotic supplementation on symptoms in functional dyspepsia[J]. Journal of Functional Foods,2020,68:103902. doi: 10.1016/j.jff.2020.103902
[18] ZHANG J, WU H M, WANG X, et al. Efficacy of prebiotics and probiotics for functional dyspepsia:A systematic review and meta-analysis[J]. Medicine,2020,99(7):19107. doi: 10.1097/MD.0000000000019107
[19] IGARASHI M, NAKAE H, MATSUOKA T, et al. Alteration in the gastric microbiota and its restoration by probiotics in patients with functional dyspepsia[J]. BMJ Open Gastroenterology,2017,4(1):144.
[20] XUE Z, WU C, WEI J, et al. An orally administered magnoloside a ameliorates functional dyspepsia by modulating brain-gut peptides and gut microbiota[J]. Life Sci,2019,233:116749. doi: 10.1016/j.lfs.2019.116749
[21] JIANG W, WU J, ZHU S, et al. The role of short chain fatty acids in irritable bowel syndrome[J]. J Neurogastroenterol Motil,2022,28(4):540−548. doi: 10.5056/jnm22093
[22] 杭露, 周盐, 孟杨杨, 等. 短链脂肪酸与肠易激综合征关系的研究进展[J]. 世界华人消化杂志,2021,29(19):1102−1109. [HANG L, ZHOU Y, MENG Y Y, et al. Research progress on the relationship between short-chain fatty acids and irritable bowel syndrome[J]. World Chinese Journal of Digestology,2021,29(19):1102−1109.] doi: 10.11569/wcjd.v29.i19.1102 HANG L, ZHOU Y, MENG Y Y, et al. Research progress on the relationship between short-chain fatty acids and irritable bowel syndrome[J]. World Chinese Journal of Digestology, 2021, 29(19): 1102−1109. doi: 10.11569/wcjd.v29.i19.1102
[23] SILVA Y P, BERNARDI A, FROZZA R L. The role of short-chain fatty acids from gut microbiota in gut-brain communication[J]. Frontiers in Endocrinology (Lausanne),2020,11:25. doi: 10.3389/fendo.2020.00025
[24] 中华人民共和国卫生部. 保健食品检验与评价技术规范实施手册[M]. 北京:清华同方电子出版社, 2003:154−158. [Ministry of Health of the People's Republic of China. Implementation manual of technical specifications for inspection and evaluation of health foods[M]. Beijing:Tsinghua Tongfang Electronic Press, 2003:154−158.] Ministry of Health of the People's Republic of China. Implementation manual of technical specifications for inspection and evaluation of health foods[M]. Beijing: Tsinghua Tongfang Electronic Press, 2003: 154−158.
[25] 张炎, 张晓旭, 甄少波, 等. GC-FID快速测定人粪便中7种短链脂肪酸含量的方法研究[J]. 实验技术与管理,2023,40(5):82−88. [ZHANG Y, ZHANG X X, ZHEN S B, et al. Study on rapid determination of short-chain fatty acids in human feces by GC-FID[J]. Experimental Technology and Management,2023,40(5):82−88.] ZHANG Y, ZHANG X X, ZHEN S B, et al. Study on rapid determination of short-chain fatty acids in human feces by GC-FID[J]. Experimental Technology and Management, 2023, 40(5): 82−88.
[26] MEI L, ZHOU J, SU Y, et al. Gut microbiota composition and functional prediction in diarrhea-predominant irritable bowel syndrome[J]. BMC Gastroenterol,2021,21(1):105. doi: 10.1186/s12876-021-01693-w
[27] CARROLL I M, RINGEL-KULKA T, SIDDLE J P, et al. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome[J]. Neurogastroenterol Motil,2012,24(6):521−530. doi: 10.1111/j.1365-2982.2012.01891.x
[28] 王菲, 高斌. 腹泻型肠易激综合征患者的肠道微生态特征[J]. 临床合理用药杂志,2021,14(8):157−158. [WANG F, GAO B. Intestinal microbiome characteristics of patients with diarrheal irritable bowel syndrome[J]. Chinese Journal of Clinical Rational Drug Use,2021,14(8):157−158.] WANG F, GAO B. Intestinal microbiome characteristics of patients with diarrheal irritable bowel syndrome[J]. Chinese Journal of Clinical Rational Drug Use, 2021, 14(8): 157−158.
[29] 裘建明, 杨关根, 王东, 等. 复杂性肛瘘患者肠道微生态检测及分析[J]. 中华胃肠外科杂志,2022,25(9):792−797. [QIU J M, YANG G G, WANG D, et al. Detection and analysis of intestinal microecology in patients with complicated fistula[J]. Chinese Journal of Gastrointestinal Surgery,2022,25(9):792−797.] doi: 10.3760/cma.j.cn441530-20220412-00142 QIU J M, YANG G G, WANG D, et al. Detection and analysis of intestinal microecology in patients with complicated fistula[J]. Chinese Journal of Gastrointestinal Surgery, 2022, 25(9): 792−797. doi: 10.3760/cma.j.cn441530-20220412-00142
[30] ZHAO L, ZHANG Q, MA W, et al. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota[J]. Food & Function,2017,8(12):4644−4656.
[31] LIU Y, LI W, YANG H, et al. Leveraging 16S rRNA microbiome sequencing data to identify bacterial signatures for irritable bowel syndrome[J]. Front Cell Infect Microbiol,2021,11:645951. doi: 10.3389/fcimb.2021.645951
[32] BHATTACHARJEE D, FLORES C, WOELFEL-MONSIVAIS C, et al. Diversity and prevalence of clostridium innocuum in the human gut microbiota[J]. MSphere,2023,8(1):56922.
[33] 潘杰, 刘来浩, 牟建伟. 肠道菌群与人类健康研究进展[J]. 山东师范大学学报(自然科学版),2021,36(4):337−365. [PAN J, LIU L H, MOU J W. Research progress on intestinal flora and human health[J]. Journal of Shandong Normal University (Natural Science),2021,36(4):337−365.] doi: 10.3969/j.issn.1001-4748.2021.04.002 PAN J, LIU L H, MOU J W. Research progress on intestinal flora and human health[J]. Journal of Shandong Normal University (Natural Science), 2021, 36(4): 337−365. doi: 10.3969/j.issn.1001-4748.2021.04.002
[34] SINGH H, TORRALBA M G, MONCERA K J, et al. Gastro-intestinal and oral microbiome signatures associated with healthy aging[J]. GeroScience,2019,41(6):907−921. doi: 10.1007/s11357-019-00098-8
[35] YI W, JI Y, GAO H, et al. Does the gut microbiome partially mediate the impact of air pollutants exposure on liver function? Evidence based on schizophrenia patients[J]. Environ Pollut,2021,291:118135. doi: 10.1016/j.envpol.2021.118135
[36] SELMA M V, BELTRAN D, LUNA M C, et al. Isolation of human intestinal bacteria capable of producing the bioactive metabolite isourolithin a from ellagic acid[J]. Front Microbiol,2017,8:1521. doi: 10.3389/fmicb.2017.01521
[37] 王慕华, 麻杰, 曹睿, 等. 腹泻患者肠道菌群特点及益生菌的干预研究[J]. 中国乳品工业,2022,50(11):11−15. [WANG M H, MA J, CAO R, et al. Intestinal microbiota characteristics of diarrhea patients and intervention study of probiotics[J]. China Dairy Industry,2022,50(11):11−15.] WANG M H, MA J, CAO R, et al. Intestinal microbiota characteristics of diarrhea patients and intervention study of probiotics[J]. China Dairy Industry, 2022, 50(11): 11−15.
[38] HARATA G, KUMAR H, HE F, et al. Probiotics modulate gut microbiota and health status in Japanese cedar pollinosis patients during the pollen season[J]. European Journal of Nutrition,2017,56(7):2245−2253. doi: 10.1007/s00394-016-1264-3
[39] 臧凯丽, 江岩, 孙勇, 等. 益生菌剂调整肠道疾病人群菌群结构丰度水平的研究[J]. 食品科学,2018,39(13):133−143. [ZANG K L, JIANG Y, SUN Y, et al. Study on the regulation of microbiota structure abundance level by probiotics in people with intestinal diseases[J]. Food Science,2018,39(13):133−143.] ZANG K L, JIANG Y, SUN Y, et al. Study on the regulation of microbiota structure abundance level by probiotics in people with intestinal diseases[J]. Food Science, 2018, 39(13): 133−143.
[40] 孙二娜, 张小妹, 赵伊凡, 等. 副干酪乳杆菌LC-37乳酸菌饮料对人体的促消化和调节肠道菌群作用[J]. 中国食品学报,2021,21(10):95−100. [SUN E N, ZHANG X M, ZHAO Y F, et al. Effect of Lactobacillus paracasei LC-37 lactobacillus beverage on promoting digestion and regulating intestinal microbiota in the human body[J]. Chinese Institute of Food Science and Technology,2021,21(10):95−100.] SUN E N, ZHANG X M, ZHAO Y F, et al. Effect of Lactobacillus paracasei LC-37 lactobacillus beverage on promoting digestion and regulating intestinal microbiota in the human body[J]. Chinese Institute of Food Science and Technology, 2021, 21(10): 95−100.
[41] YANG B, YUE Y, CHEN Y, et al. Lactobacillus plantarum CCFM1143 alleviates chronic diarrhea via inflammation regulation and gut microbiota modulation:A double-blind, randomized, placebo-controlled study[J]. Front Immunol,2021,12:746585. doi: 10.3389/fimmu.2021.746585
[42] 蒙丹丽, 梁列新, 宋怀宇. 短链脂肪酸在肠道中的生理作用[J]. 中国临床新医学,2018,11(2):198−202. [MENG D L, LIANG L X, SONG H Y. Physiological role of short-chain fatty acids in intestinal tract[J]. Chinese Journal of Clinical New Medicine,2018,11(2):198−202.] MENG D L, LIANG L X, SONG H Y. Physiological role of short-chain fatty acids in intestinal tract[J]. Chinese Journal of Clinical New Medicine, 2018, 11(2): 198−202.
[43] 张浩. 肠易激综合征和炎症性肠病患者肠道优势菌群特征及其与疾病发生相关性研究[D]. 南京:南京大学, 2011. [ZHANG H. Characteristics of dominant intestinal flora in patients with irritable bowel syndrome and inflammatory bowel disease and its correlation with disease occurrence[D]. Nanjing:Nanjing University, 2011.] ZHANG H. Characteristics of dominant intestinal flora in patients with irritable bowel syndrome and inflammatory bowel disease and its correlation with disease occurrence[D]. Nanjing: Nanjing University, 2011.
[44] PAULINA M, KATARZYNA Ś. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome[J]. Nutrients,2020,12(4):1107. doi: 10.3390/nu12041107
[45] 高文文, 孟祥晨. 人肠道产丁酸细菌及其所产丁酸的促健康作用研究进展[J]. 食品科学,2019,40(21):273−279. [GAO W W, MENG X C. Research progress on human intestinal butyric acid-producing bacteria and the health-promoting effects of butyric acid produced[J]. Food Science,2019,40(21):273−279.] GAO W W, MENG X C. Research progress on human intestinal butyric acid-producing bacteria and the health-promoting effects of butyric acid produced[J]. Food Science, 2019, 40(21): 273−279.
[46] BELENGUER A, DUNCAN S H, CALDER A G, et al. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut[J]. Appl Environ Microbiol,2006,72(5):3593−3599. doi: 10.1128/AEM.72.5.3593-3599.2006





 下载:
下载:



 下载:
下载:
