Medicinal and Nutritional Value of the Chemical Compositions of Dioscorea opposita Thunb. cv. Tiegun Peel Based on UPLC-Q/TOF-MS/MS and Bioinformatics
-
摘要: 目的:系统分析并识别出铁棍山药皮中的化学成分,探讨其药用和营养价值。方法:采用超高效液相色谱-飞行时间串联质谱技术(UPLC-Q/TOF-MS/MS)对铁棍山药皮中的化学成分进行分析,根据化合物一级、二级质谱信息,与参考文献和数据库进行比对,确认所含化合物。利用生物信息学方法,对潜在活性成分进行靶点预测、京都基因与基因组百科全书(KEGG)通路富集分析,发挥作用的重要靶点筛选以及其作用机制的探讨。结果:从铁棍山药皮中共鉴别出33种化合物,其中芹菜素、金合欢素、甘草素、山药素I等化合物为铁棍山药皮中潜在的发挥功效的重要化合物,通路富集分析表明铁棍山药皮主要作用于细胞信号通路、癌症和代谢类等疾病相关通路。结论:本研究基于UPLC-Q/TOF-MS/MS结合生物信息学方法,为铁棍山药皮的成分识别、活性物质筛选、潜在药用和营养价值及进一步的开发利用提供了重要参考。
-
关键词:
- 铁棍山药皮 /
- 超高效液相色谱-飞行时间串联质谱 /
- 化学成分 /
- 生物信息学 /
- 作用机制
Abstract: Objective: Systematic analysis and identification of chemical constituents of Dioscorea opposita Thunb. cv. Tiegun peel, and exploration of its medicinal and nutritional value. Methods: Ultra-high performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (UPLC-Q/TOF-MS/MS) was performed to analyze the chemical compositions of Dioscorea opposita Thunb. cv. Tiegun peel. The compounds were identified based on their primary and secondary mass spectrometry information and compared with the related references and databases. Further, bioinformatics was applied to predict the targets of potential active ingredients, enrich Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, screen out the important targets, and explore its potential mechanism. Results: 33 compounds were identified such as apigenin, acacetin, liquiritigenin, batatasin I, which were considered to be the potentially vital compounds. Pathway enrichment analysis indicated that Dioscorea opposita Thunb. cv. Tiegun peel exerted medicinal effects by regulating cell signaling pathways, cancer, metabolic and other disease-related pathways. Conclusion: In this study, UPLC-Q/TOF-MS/MS combined with bioinformatics were used, which would provide an important reference for the identification of ingredients, selection of active compounds, discovery of potential medicinal nutritional value, as well as further development and utilization of Dioscorea opposita Thunb. cv. Tiegun peel. -
铁棍山药(Dioscorea opposite Thunb. cv. Tiegun)为薯蓣科植物薯蓣的块茎,分布于河南省武陟县、温县等地的山药品种,其性平味甘,健脾止泻、补肺益肾,具有“补虚羸、除寒邪热、补中益气力、长肌肉、久服耳目聪明、轻身不饥、延年”等功效,属山药中的极品[1]。铁棍山药富含多糖、蛋白、脂类、黄酮、多酚类等多种活性成分,具有调节脾胃、增强免疫、抗氧化、抗肿瘤、抗衰老等作用,兼有食用价值和药用价值[2-3]。
除了铁棍山药的药用饮片外,目前市场上也不断涌现出以铁棍山药为原料制成的零食或保健品,如铁棍山药薯片、铁棍山药饼干、铁棍山药粉等。然而,无论是在直接食用或加工铁棍山药过程中,铁棍山药皮一般都直接削除丢弃,造成了极大的资源浪费。目前,关于铁棍山药皮的报道尚少,仅见对其所含部分成分的研究,如多糖[4]、黄酮类化合物的提取[5]以及多酚化合物的体外抗氧化作用[6]。这些研究仅关注单类化合物,对其所含化学成分的认识不够全面,无法反映其整体的化学成分特征。此外,铁棍山药皮药用和营养价值尚不明确,这也严重阻碍了其再利用。因此,全面、系统识别铁棍山药皮所含的化学成分,并挖掘其相应的分子机制,对于铁棍山药皮活性功能物质的开发应用具有重要的指导意义。
超高效液相色谱-四极杆-飞行时间串联质谱(UPLC-Q/TOF-MS/MS)具有速度快、灵敏度高、分辨率高等特点[7],根据化合物质谱信息,结合相关数据库,能够对中草药、复方制剂、天然产物等中的化学成分进行快速分离和鉴定。生物信息学是综合运用系统生物学、计算机科学、信息技术和数学理论等技术方法的交叉学科[8],能够从中草药、复方制剂、天然产物机制研究、复杂疾病治疗等生命科学的海量数据中,发现、挖掘、阐述其中所包含生物学意义[9]。
联合UPLC-Q/TOF-MS/MS和生物信息学的方法越来越多地应用于多成分多靶点多通路的机制研究[10-11]。本研究拟采用UPLC-Q/TOF-MS/MS技术结合质谱数据库对铁棍山药皮中的化学成分进行定性识别,并在此基础上,利用生物信息学对其潜在的靶点进行预测和功能分析,探讨铁棍山药皮化合物成分的药用和营养价值,为铁棍山药皮的开发利用提供科学依据。
1. 材料与方法
1.1 材料与仪器
山药皮 河南省温县铁棍山药的山药皮;甲醇、乙腈 色谱级,美国EMD Millipore公司;甲酸 色谱级,美国Sigma公司。
Exion 20AD型超高效液相色谱仪、X500R Q-TOF型高分辨质谱仪(SCIEX OS软件) 美国SCIEX公司;ME204E电子分析天平 瑞士梅特勒-托利多公司;Milli-Q Synthesis 超纯水系统 美国Millipore 公司;DW-HL398S超低温冷藏冰箱 中科美菱低温科技股份有限公司。
1.2 实验方法
1.2.1 样品制备
将铁棍山药流水清洗干净,晾干,削皮后即刻将皮置于−80 ℃环境中过夜冷冻,取出后迅速于冷冻干燥机内−90 ℃真空低温冷冻干燥,待完全干燥后制成铁棍山药皮冻干粉,过8号筛。称取1.0 g,加入5 mL甲醇:水=1:1(v/v)超声提取30 min,在10000 r/min的条件下离心10 min,吸取上清液进样[12]。
1.2.2 色谱条件
Waters HSS T3色谱柱(100×3.0 mm,1.8 µm);流动相:0.05%甲酸水(A)-乙腈(B),梯度洗脱程序为:0~1.0 min,3% B;1.0~10 min,3%~30% B;10~20 min,30%~99% B;20~26 min,99% B;26~26.1 min,99%~3% B;26.1~30 min,3% B。进样盘温度:15 ℃;流速:0.40 mL/min;柱温:40 ℃。
1.2.3 质谱条件
质谱采用电喷雾离子源ESI检测,在正、负离子模式下进行样品采集,开启动态背景以扣除干扰。相关参数设置为:气帘气流(CUR)25 psi;雾化气流速(GS1)50 psi;辅助气流速(GS2)50 psi;离子源电压(ISVF)5500/−4500 V;离子源温度(TEM)500 ℃;捕获电压(DP)80 V;碰撞电压(CE)35±15 V。一级质谱m/z扫描范围设为50~1300 Da,扫描累计时间0.25 s,选择响应最高的碎片进行二级扫描,m/z扫描范围设为50~1000 Da,扫描累计时间0.1 s。
1.2.4 化合物鉴定
采用SCIEX OS软件进行质谱原始数据的采集和处理,通过质量准确度、同位素丰度、二级谱库确认及保留时间等,根据SCIEX自建天然产物数据库SCIEX TCM Library自动进行峰提取和筛查,进一步根据获得的质谱信息结合参考文献、有机小分子生物活性数据库(PubChem: https://pubchem.ncbi.nlm.nih.gov/)[13]、人类代谢组数据库(HMDB:https://hmdb.ca/)[14]和植物活性分子数据库(Collective Molecular Activities of Useful Plants: CMAUP)[15]进行铁棍山药皮中化合物的鉴定识别。
1.2.5 活性成分筛选
通过中药系统药理学数据库与分析平台(Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform,TCMSP)将所鉴别的化合物成分进行口服生物利用度(oral bioavailability,OB)和药物相似度(drug-likeness,DL)的查找。根据TCMSP数据库分析平台筛选标准[16]OB≥20%和DL≥0.1筛选出潜在活性成分。
1.2.6 铁棍山药皮-化合物-靶点网络构建
将筛选出的活性成分利用PubChem数据库找到相应的SMILES信息,通过SwissTargetPrediction数据库(http://www.swisstargetprediction.ch/),设定物种“Homo sapiens”进行检索,选择Probability值大于0的值,获取其潜在的作用靶标[17],利用Cytoscape 3.8.2分析软件构建铁棍山药皮-化合物-靶点相互关系网络。
1.2.7 潜在作用靶点功能富集分析
将1.2.6项下获得的作用靶点,通过R软件中的ClusterProfiler软件包进行相关靶点的KEGG通路分析[18],以P<0.05为筛选条件,获得铁棍山药皮中目标化合物富集的相关通路,进而分析其药理和营养作用机理。
2. 结果与分析
2.1 基于UPLC-Q/TOF-MS/MS平台的铁棍山药皮中化学成分识别
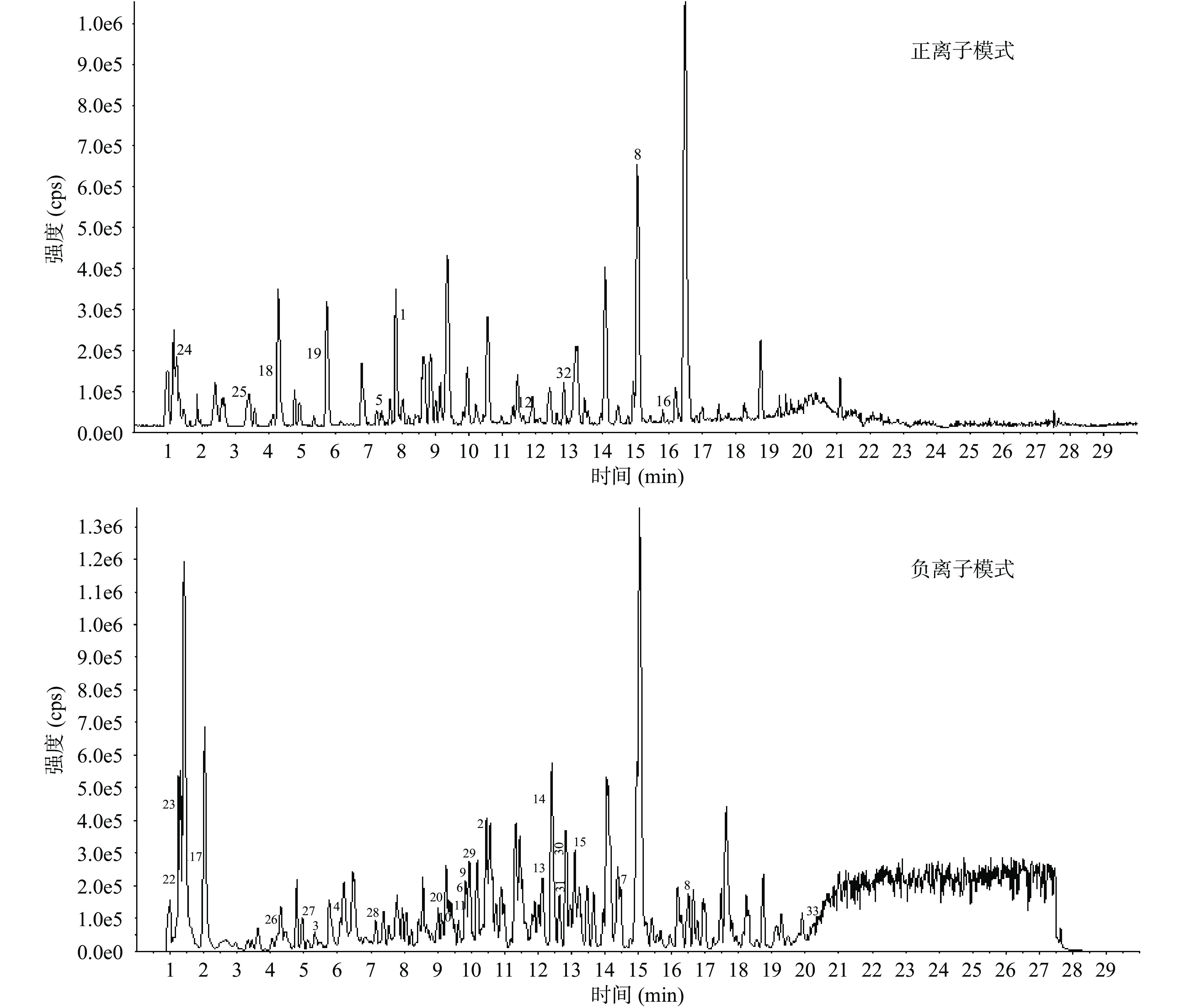
采用上述色谱和质谱条件对铁棍山药皮提取物中的化学成分进行检测,其正、负离子模式扫描下质谱基峰离子流图如图1所示。利用SCIEX OS软件拟合计算可能的分子式,结合本地SCIEX TCM Library数据库对铁棍山药皮质谱离子流图匹配出的化合物进行前期推测,再根据参考文献、CMAUP、PubChem、HMDB等数据库提供的一级、二级离子信息等进行进一步确定,最终鉴别出33种化合物,其中酚酸类9种,黄酮类7种,氨基酸有机酸类5种,糖类2种及其他类10种,以上成分相关归属及鉴定信息见表1。
表 1 铁棍山药皮提取物中化学成分的鉴定Table 1. Identification of chemical composition of Dioscorea opposita Thunb. cv. Tiegun peel分
类编号 中文名称 英文名称 分子式 相对分子
质量保留时间
(min)MS MS/MS 模式 偏差
(ppm)参考文献 酚酸类 1 儿茶素 Catechin C15H14O6 290.079 7.959 291.087 139.0294,123.0358,
147.0340P 0.3 [13−14,
19−21]2 表儿茶素 Epicatechin C15H14O6 290.079 10.446 289.0711 243.0695,175.0788,
159.0472N 0.1 [13−14,
19−21]3 葡萄糖基丁香酸 Glucosyringic Acid C15H20O10 360.1056 5.383 359.0974 197.0474,138.0349,
182.0246N 0.1 [13−14,22] 4 原花青素 B1 Procyanidin B1 C30H26O12 578.1424 6.174 577.1345 407.0840,289.0754,
125.0266N −1 [13−14,19] 5 原花青素 B2 Procyanidin B2 C30H26O12 578.1424 7.361 579.1506 127.0303,287.0348,
409.0628,427.0724P 0.5 [13,19] 6 丁香酸 Syringic acid C9H10O5 198.0528 9.777 197.0448 182.0239,167.0005,
123.0100N −0.5 [13−14,
22−23]7 山药素III Batatasin III C15H16O3 244.1099 14.516 243.102 228.0780,183.0840 N −2 [13−15,
20−21]8 山药素I Batatasin I C17H16O4 284.1049 16.531 283.0969 253.0540,267.0703,
197.0627N −1 [13−15] 9 阿魏酸 Ferulic acid C10H10O4 194.0579 9.896 193.0491 178.0302,134.0393 N −5 [13−14,24] 黄酮类 10 芦丁 Rutin C27H30O16 610.1534 9.337 609.1449 301.0409,300.0331,
189.0054N −1 [13−14,23] 11 异槲皮素 Isoquercetin C21H20O12 464.0955 9.684 463.0873 463.0958,301.0393,
271.0283,255.0328N −0.6 [13−14,21] 12 芹菜素 Apigenin C15H10O5 270.0528 11.649 270.0528 228.0265,243.0496,
187.0264P 0.7 [13−15,21] 13 异落叶松脂素 Isolariciresinol C20H24O6 360.1573 12.387 359.1482 313.1318,165.0574 N −0.3 [13−15] 14 甘草素 Liquiritigenin C15H12O4 256.0736 12.459 255.0654 240.0450,183.0469,
211.0423,241.0489N 1 [13−14,21,25] 15 金合欢素 Acacetin C16H12O5 284.0685 13.183 283.0604 183.0473,211.0429,
240.0455N −0.7 [13,15] 16 去二甲氧
基姜黄素Bisdemethoxycurcumin C19H16O4 308.1049 15.747 309.1129 147.0338,225.0705, P 0.3 [13−14,26] 氨基酸
有机酸类17 柠檬酸 Citric Acid C6H8O7 192.027 1.948 191.0188 111.0116,129.0208 N 0.1 [13−14,22] 18 L-苯丙氨酸 L-Phenylalanine C9H11NO2 165.079 4.273 166.0866 120.0721,103.0470,
91.0484P 0.6 [13−14,22] 19 L-色氨酸 L-Tryptophan C11H12N2O2 204.0899 5.742 205.0975 118.0570,146.0497,
143.0629P −1 [13−15] 20 N-乙酰基-L-
苯丙氨酸N-Acetyl-L-
phenylalanineC11H13NO3 207.0895 9.112 206.0815 164.0742,147.0473,
91.0591,206.0847N 0.5 [13−14,22] 21 L-亮氨酸 L-Leucine C6H13NO2 131.0946 27.852 132.1016 72.9332,55.9323,
90.8991P −2 [13−15] 糖类 22 果糖 D-Fructose C6H12O6 180.0634 1.169 179.0556 59.0200,71.0180,
89.0268,113.0268,
101.0279N 0.6 [13−14,22] 23 D-蔗糖 D-Sucrose C12H22O11 342.1162 1.268 341.1081 179.0577,119.0372,
89.0276N −0.6 [13−14,22] 其他类 24 尿囊素 Allantoin C4H6N4O3 158.044 1.227 159.0517 61.0374,99.0127,
73.0356P 1 [13−14,21−22] 25 腺苷 Adenosine C10H13N5O4 267.0968 3.391 268.1048 136.0523,119.0273 P 0.7 [13−14,21−22] 26 1-没食子酸
酰葡萄糖1-Galloylglucose C13H16O10 332.07435 4.287 331.0664 168.0083,125.0265 N −0.3 [13,27] 27 D-泛酸 D-Pantothenic Acid C9H17NO5 219.1107 4.968 218.1025 146.0838,88.0435 N 0.1 [13−14,22] 28 杜仲酸 Eucomic acid C11H12O6 240.0634 7.164 239.0553 179.0369,177.0580,
149.0630N 0.4 [13,15] 29 葛根内酯A Kuzubutenolide A C23H24O10 460.1369 9.977 459.1294 233.0488,339.0930,
205.0534N 3 [13,27] 30 汉诺醇 Hannokinol C19H24O4 316.1675 12.599 315.1591 149.0629,147.0479 N 2 [13,15] 31 苦木内酯I Nigakilactone I C21H28O6 376.1886 12.813 375.1806 135.0475,179.0737 N −0.5 [13,15] 32 迷迭香双醛 Rosmadial C20H24O5 344.1624 12.898 345.1705 177.0788,137.0505 P 0.6 [13−15] 33 香草酸 Vanillic acid C8H8O4 168.0423 20.371 167.0344 123.0104,69.0001 N 3.6 [13−14,28] 注:P为正离子模式;N为负离子模式。 2.2 活性成分筛选
将铁棍山药皮中鉴别所获得的化合物成分,根据TCMSP数据库分析平台筛选标准OB≥20%和DL≥0.1筛选出潜在活性成分10个,见表2。
表 2 铁棍山药皮中通过OB和DL预测的10个活性成分Table 2. Ten active ingredients predicted by OB and DL in Dioscorea opposita Thunb. cv. Tiegun peel分子ID 中文名称 OB(%) DL MOL000004 原花青素B1 67.87 0.66 MOL000008 芹菜素 23.06 0.21 MOL000073 表儿茶素 48.96 0.24 MOL000096 儿茶素 49.68 0.24 MOL000940 去二甲氧基姜黄素 77.38 0.26 MOL001689 金合欢素 34.97 0.24 MOL001792 甘草素 32.76 0.18 MOL002323 腺苷 19.85 0.16 MOL005443 山药素I 23.7 0.27 MOL007925 葡萄糖基丁香酸 24.29 0.30 2.3 潜在作用靶标预测和网络构建
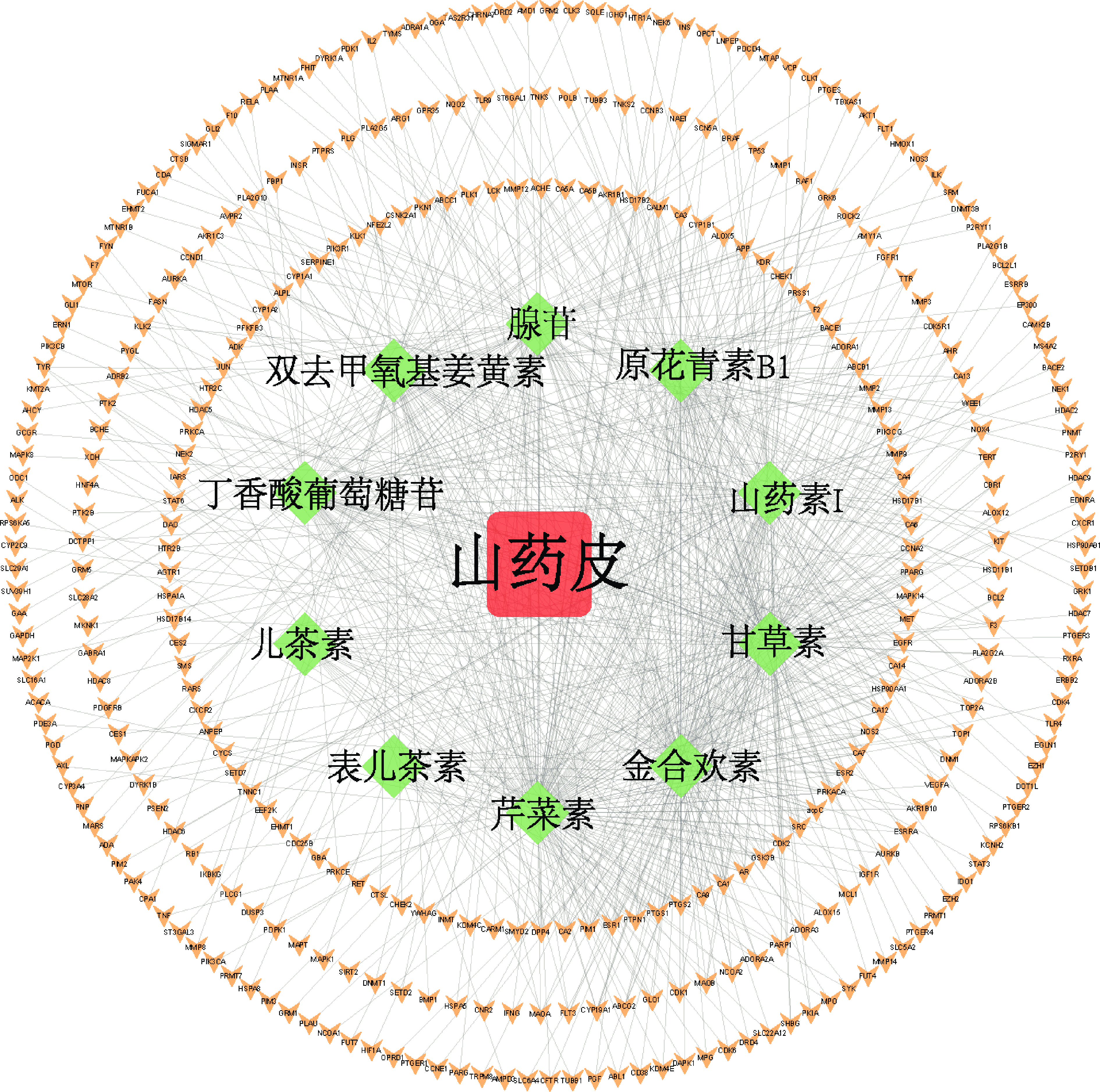
进一步通过SwissTargetPrediction数据库对获得的10个化合物靶点进行预测,去掉重复基因名称,共获得348个潜在作用靶点。利用Cytoscape 3.8.2软件构建出铁棍山药皮-化合物-靶点网络,见图2。
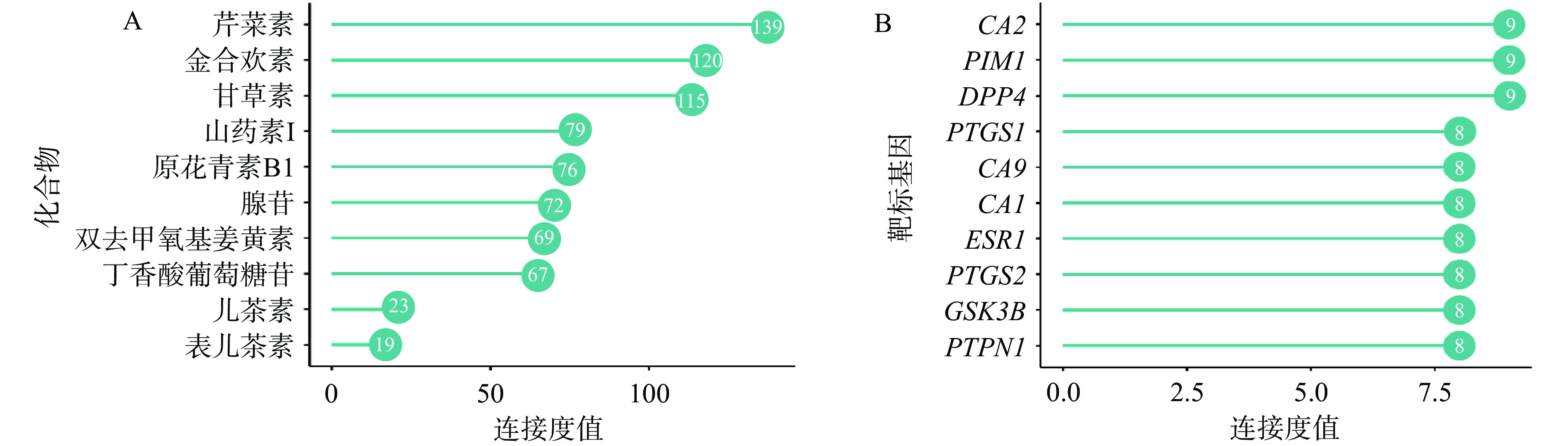
网络中连接度值表明此化合物或靶点的重要程度,化合物的连接度分析发现,10个化合物中芹菜素、金合欢素、甘草素、山药素I、原花青素B1、腺苷、双去甲基姜黄素和丁香酸葡萄糖苷这8个化合物的连接度值均在60以上,其中芹菜素、金合欢素和甘草素的连接度值均大于100(图3A),上述化合物可能为铁棍山药皮中潜在发挥功效的重要化合物;碳酸酐酶2(CA2)、丝/苏氨酸蛋白激酶Pim-1(PIM1)、二肽基肽酶4(DPP4)、环加氧酶1(PTGS1)、碳酸酐酶9(CA9)、碳酸酐酶1(CA1)、雌激素受体α(ESR1)、环加氧酶 2(PTGS2)、糖原合成酶激酶3β(GSK3B)和蛋白质酪氨酸磷酸1(PTPN1)为连接度排名前10的作用靶点(图3B),其可能为铁棍山药皮作用的主要靶点。
2.4 潜在作用靶点KEGG功能富集分析
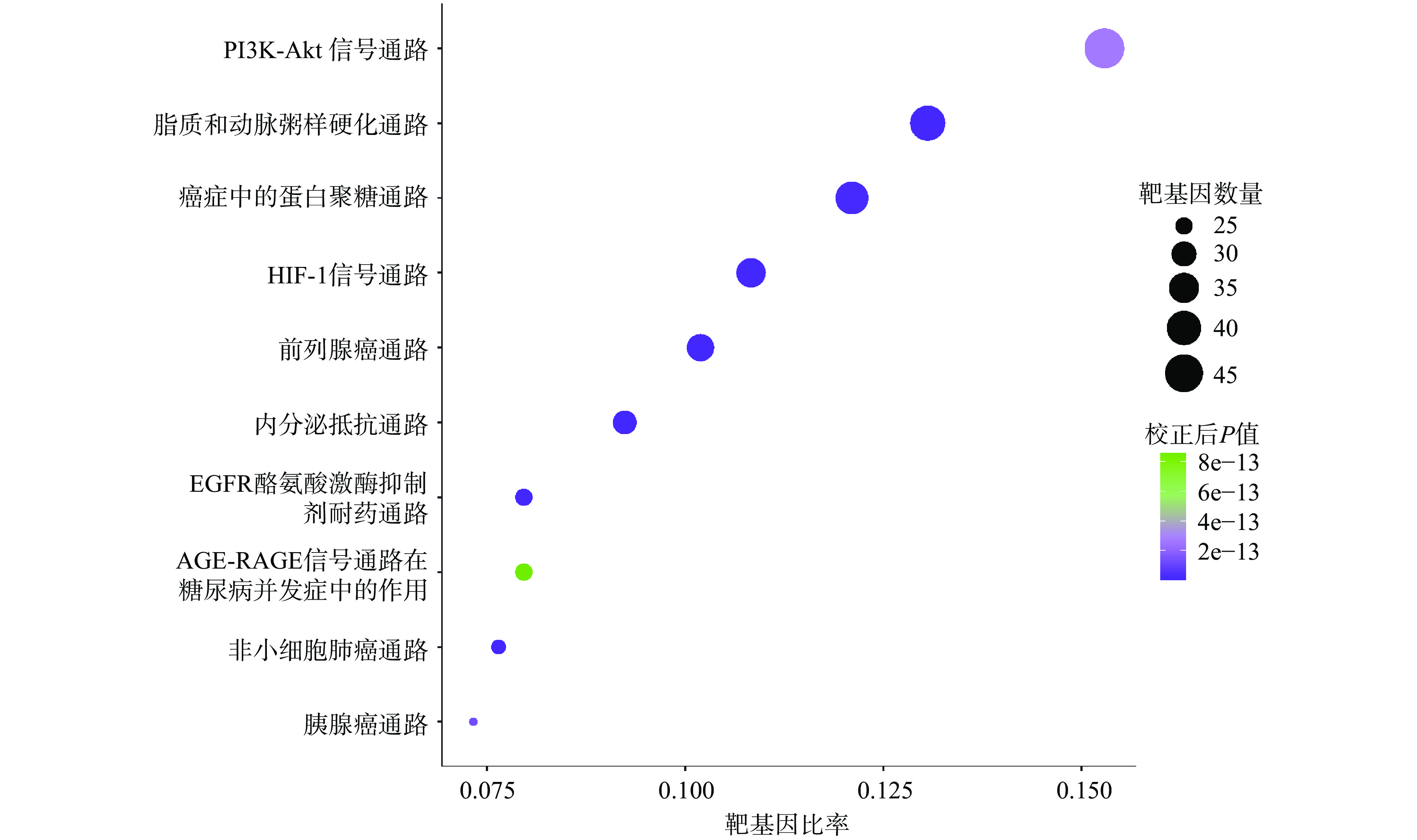
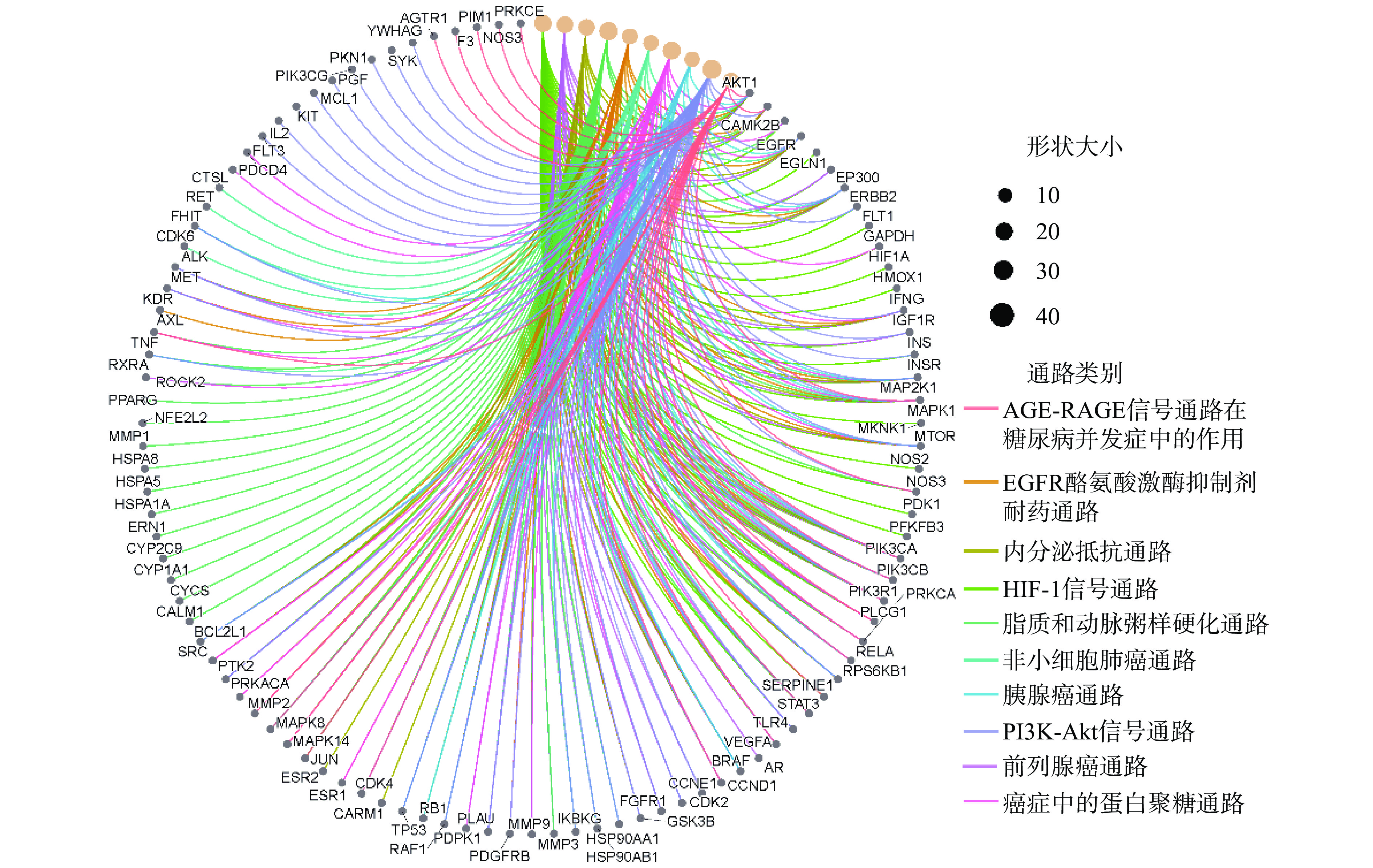
将获得的潜在作用靶基因通过R软件中的ClusterProfiler软件包进行KEGG功能富集分析,其中富集程度最高且排名前10条重要代谢通路分别为:PI3K-Akt信号通路(PI3K-Akt signaling pathway)、脂质和动脉粥样硬化通路(Lipid and atherosclerosis)、癌症蛋白多糖信号通路(Proteoglycans in cancer)、HIF-1信号通路(HIF-1 signaling pathway)、前列腺癌(Prostate cancer)、内分泌抵抗(Endocrine resistance)、EGFR酪氨酸激酶抑制剂耐药性(EGFR tyrosine kinase inhibitor resistance)、AGE-RAGE信号通路在糖尿病并发症中的作用信号通路(AGE-RAGE signaling pathway in diabetic complications)、非小细胞肺癌通路(Non-small cell lung cancer)和胰腺癌通路(Pancreatic cancer),见图4。
KEGG通路富集分析显示,铁棍山药皮活性成分对应的通路主要集中在细胞信号通路、癌症和代谢类疾病治疗等方面。其中,在细胞信号通路上,分别有48和34个基因富集在PI3K-Akt信号通路和HIF-1信号通路;抗癌症方面,分别有38、32、25、24和23个基因富集在癌症蛋白多糖信号通路、前列腺癌通路、EGFR酪氨酸激酶抑制剂耐药通路、非小细胞肺癌通路和胰腺癌通路等通路上面;调节代谢系统方面,分别41、25和29个基因富集在脂质和动脉粥样硬化通路、AGE-RAGE信号通路在糖尿病并发症中的作用信号通路和内分泌抵抗通路。图5展示了这些KEGG通路与靶基因之间的相互关系。
3. 讨论与结论
针对铁棍山药中化合物成分及其药理活性的研究较多,相对而言,铁棍山药皮的相关研究报道较少[4-6]。本文利用UPLC-Q/TOF-MS/MS平台,分析并鉴别出了铁棍山药皮中的33种化合物,为其成分的识别和潜在价值奠定了前期基础;通过生物信息学分析方法,找出铁棍山药皮中发挥功效的关键化学成分,其中芹菜素、金合欢素、甘草素、去二甲氧基姜黄素、山药素I等物质具有良好的生物活性。如芹菜素具有抗炎、抗肿瘤侵袭和转移、保护肝功能、抗动脉硬化和脑血栓、降压和镇静抗焦虑等功效[29],金合欢素具有抗肿瘤、免疫调节、保护心脏和神经系统等多种药理活性[30],甘草素具有良好的抗癌活性和抗肿瘤作用[31],去二甲氧基姜黄素具有抗细胞凋亡、抗氧化和抗炎作用[32],山药素I具有良好的抗炎作用[33],由此可见,铁棍山药皮可能通过多成分共同发挥其药用和营养价值。同时,这些功能成分的鉴别对于铁棍山药皮中营养与药用功能物质的开发和利用也有着重要的参考意义。
铁棍山药皮-化合物-靶点网络研究发现,铁棍山药皮具有多个作用靶点,其中重要潜在靶点包括癌症肿瘤相关基因(PIM1)、治疗糖尿病代谢相关基因(GSK3B、PTPN1、DPP4)、雌激素受体(ESR1)、环加氧酶(PTGS1、PTGS2)和多种碳酸酐酶(CA2、CA9、CA1)等,这些基因与癌症肿瘤、糖尿病、细胞内信号传导、细胞生长和增殖调控及代谢等密切相关,体现了铁棍山药皮多成分-多靶点系统调节的作用机制。研究将成分对应的靶基因进行KEGG富集分析,明确了铁棍山药皮成分靶点对应的疾病主要集中在肿瘤癌症、细胞信号传导、糖尿病及代谢类等相关途径,这一发现也为药物的进一步研发提供了科学依据。
本研究采用UPLC-Q/TOF-MS/MS分析技术进行了铁棍山药皮中化学成分的鉴别,通过生物信息学预测了潜在活性化合物具有的多种药理活性,初步明确了铁棍山药皮具有的药用和营养价值,但铁棍山药皮尚需进一步深入挖掘和研究。首先,对铁棍山药皮中化合物成分的提取工艺、定性和定量分析研究十分必要;其次,本文通过生物信息学技术建立的山药皮-化合物-靶点网络中,所涉及的重要化合物和重要靶点需深入研究;最后,铁棍山药皮的再利用过程,需要建立规范化流程,进而可以形成产品,有望作为保健品或者中医药产品应用于人类疾病的预防和治疗。由此可见,本研究为铁棍山药皮资源的有效利用提供了物质基础和开发依据,也为进一步延长铁棍山药的产业链、提高其附加值提供了理论数据。
-
表 1 铁棍山药皮提取物中化学成分的鉴定
Table 1 Identification of chemical composition of Dioscorea opposita Thunb. cv. Tiegun peel
分
类编号 中文名称 英文名称 分子式 相对分子
质量保留时间
(min)MS MS/MS 模式 偏差
(ppm)参考文献 酚酸类 1 儿茶素 Catechin C15H14O6 290.079 7.959 291.087 139.0294,123.0358,
147.0340P 0.3 [13−14,
19−21]2 表儿茶素 Epicatechin C15H14O6 290.079 10.446 289.0711 243.0695,175.0788,
159.0472N 0.1 [13−14,
19−21]3 葡萄糖基丁香酸 Glucosyringic Acid C15H20O10 360.1056 5.383 359.0974 197.0474,138.0349,
182.0246N 0.1 [13−14,22] 4 原花青素 B1 Procyanidin B1 C30H26O12 578.1424 6.174 577.1345 407.0840,289.0754,
125.0266N −1 [13−14,19] 5 原花青素 B2 Procyanidin B2 C30H26O12 578.1424 7.361 579.1506 127.0303,287.0348,
409.0628,427.0724P 0.5 [13,19] 6 丁香酸 Syringic acid C9H10O5 198.0528 9.777 197.0448 182.0239,167.0005,
123.0100N −0.5 [13−14,
22−23]7 山药素III Batatasin III C15H16O3 244.1099 14.516 243.102 228.0780,183.0840 N −2 [13−15,
20−21]8 山药素I Batatasin I C17H16O4 284.1049 16.531 283.0969 253.0540,267.0703,
197.0627N −1 [13−15] 9 阿魏酸 Ferulic acid C10H10O4 194.0579 9.896 193.0491 178.0302,134.0393 N −5 [13−14,24] 黄酮类 10 芦丁 Rutin C27H30O16 610.1534 9.337 609.1449 301.0409,300.0331,
189.0054N −1 [13−14,23] 11 异槲皮素 Isoquercetin C21H20O12 464.0955 9.684 463.0873 463.0958,301.0393,
271.0283,255.0328N −0.6 [13−14,21] 12 芹菜素 Apigenin C15H10O5 270.0528 11.649 270.0528 228.0265,243.0496,
187.0264P 0.7 [13−15,21] 13 异落叶松脂素 Isolariciresinol C20H24O6 360.1573 12.387 359.1482 313.1318,165.0574 N −0.3 [13−15] 14 甘草素 Liquiritigenin C15H12O4 256.0736 12.459 255.0654 240.0450,183.0469,
211.0423,241.0489N 1 [13−14,21,25] 15 金合欢素 Acacetin C16H12O5 284.0685 13.183 283.0604 183.0473,211.0429,
240.0455N −0.7 [13,15] 16 去二甲氧
基姜黄素Bisdemethoxycurcumin C19H16O4 308.1049 15.747 309.1129 147.0338,225.0705, P 0.3 [13−14,26] 氨基酸
有机酸类17 柠檬酸 Citric Acid C6H8O7 192.027 1.948 191.0188 111.0116,129.0208 N 0.1 [13−14,22] 18 L-苯丙氨酸 L-Phenylalanine C9H11NO2 165.079 4.273 166.0866 120.0721,103.0470,
91.0484P 0.6 [13−14,22] 19 L-色氨酸 L-Tryptophan C11H12N2O2 204.0899 5.742 205.0975 118.0570,146.0497,
143.0629P −1 [13−15] 20 N-乙酰基-L-
苯丙氨酸N-Acetyl-L-
phenylalanineC11H13NO3 207.0895 9.112 206.0815 164.0742,147.0473,
91.0591,206.0847N 0.5 [13−14,22] 21 L-亮氨酸 L-Leucine C6H13NO2 131.0946 27.852 132.1016 72.9332,55.9323,
90.8991P −2 [13−15] 糖类 22 果糖 D-Fructose C6H12O6 180.0634 1.169 179.0556 59.0200,71.0180,
89.0268,113.0268,
101.0279N 0.6 [13−14,22] 23 D-蔗糖 D-Sucrose C12H22O11 342.1162 1.268 341.1081 179.0577,119.0372,
89.0276N −0.6 [13−14,22] 其他类 24 尿囊素 Allantoin C4H6N4O3 158.044 1.227 159.0517 61.0374,99.0127,
73.0356P 1 [13−14,21−22] 25 腺苷 Adenosine C10H13N5O4 267.0968 3.391 268.1048 136.0523,119.0273 P 0.7 [13−14,21−22] 26 1-没食子酸
酰葡萄糖1-Galloylglucose C13H16O10 332.07435 4.287 331.0664 168.0083,125.0265 N −0.3 [13,27] 27 D-泛酸 D-Pantothenic Acid C9H17NO5 219.1107 4.968 218.1025 146.0838,88.0435 N 0.1 [13−14,22] 28 杜仲酸 Eucomic acid C11H12O6 240.0634 7.164 239.0553 179.0369,177.0580,
149.0630N 0.4 [13,15] 29 葛根内酯A Kuzubutenolide A C23H24O10 460.1369 9.977 459.1294 233.0488,339.0930,
205.0534N 3 [13,27] 30 汉诺醇 Hannokinol C19H24O4 316.1675 12.599 315.1591 149.0629,147.0479 N 2 [13,15] 31 苦木内酯I Nigakilactone I C21H28O6 376.1886 12.813 375.1806 135.0475,179.0737 N −0.5 [13,15] 32 迷迭香双醛 Rosmadial C20H24O5 344.1624 12.898 345.1705 177.0788,137.0505 P 0.6 [13−15] 33 香草酸 Vanillic acid C8H8O4 168.0423 20.371 167.0344 123.0104,69.0001 N 3.6 [13−14,28] 注:P为正离子模式;N为负离子模式。 表 2 铁棍山药皮中通过OB和DL预测的10个活性成分
Table 2 Ten active ingredients predicted by OB and DL in Dioscorea opposita Thunb. cv. Tiegun peel
分子ID 中文名称 OB(%) DL MOL000004 原花青素B1 67.87 0.66 MOL000008 芹菜素 23.06 0.21 MOL000073 表儿茶素 48.96 0.24 MOL000096 儿茶素 49.68 0.24 MOL000940 去二甲氧基姜黄素 77.38 0.26 MOL001689 金合欢素 34.97 0.24 MOL001792 甘草素 32.76 0.18 MOL002323 腺苷 19.85 0.16 MOL005443 山药素I 23.7 0.27 MOL007925 葡萄糖基丁香酸 24.29 0.30 -
[1] 苏敬. 新修本草[M]. 合肥: 安徽科学技术出版社, 1981: 150 SU J. Newly revised materia medica[M]. Hefei: Anhui Science and Technology Press, 1981: 150.
[2] CHENG Z, HU M, TAO J, et al. The protective effects of Chinese yam polysaccharide against obesity-induced insulin resistance[J]. Journal of Functional Foods,2019,55:238−247. doi: 10.1016/j.jff.2019.02.023
[3] 陈佳希. 铁棍山药有效成分提取分离及其活性研究[D]. 西安: 西北大学, 2011 CHEN X J. Extract of effective components and bioactivity research in Dioscorea opposite Thunb. cv. Tiegun[D]. Xi'an: Northwest University, 2011.
[4] 吴金松, 王晓芳, 王建玲, 等. 超声波辅助提取铁棍山药皮水溶性多糖的工艺优化[J]. 粮食与油脂,2021,34(7):110−114. [WU J S, WANG X F, WANG J L, et al. Optimization of ultrasound-assisted extraction of water-soluble polysaccharides from Chinese yam bark[J]. Cereals & Oils,2021,34(7):110−114. doi: 10.3969/j.issn.1008-9578.2021.07.028 [5] 赵立庭, 赵华, 刘阳, 等. 铁棍山药皮中黄酮化合物提取工艺条件优化[J]. 粮油食品科技,2016,24(4):59−63. [HAO L T, ZHAO H, LIU Y, et al. Optimization of extract process conditions for flavonoids in the peel of Henan tiegun yam[J]. Science and Technology of Cereals, Oils and Foods,2016,24(4):59−63. doi: 10.3969/j.issn.1007-7561.2016.04.014 [6] 孟月丽, 张庆岭. 铁棍山药皮中多酚类化合物体外抗氧化作用研究[J]. 中医学报,2016,31(5):707−710. [MENG Y L, ZHANG Q L. Anti-oxidation effect of polyphenolic compounds in Chinese yam peel in vitro[J]. China Journal of Chinese Medicine,2016,31(5):707−710. [7] ALI, JAVED, KHAN, et al. UHPLC/Q-TOF-MS technique: Introduction and applications[J]. Letters in Organic Chemistry,2015(12):371−378.
[8] 王可鉴, 贺林, 杨仑. 生物信息学在药物研究和开发中的应用[J]. 中国药理学与毒理学杂志,2014,28(1):118−125. [WANG K J, HE L, YANG L. Application of bioinformatics in pharmaceutical research and development[J]. Chinese Journal of Pharmacology and Toxicology,2014,28(1):118−125. doi: 10.3867/j.issn.1000-3002.2014.01.018 [9] 王程成, 封亮, 刘丹, 等. 结合生物信息学的中药组分结构研究思路[J]. 中国中药杂志,2015,40(22):4514−4519. [WANG C L, FENG L, LIU D, et al. Research thoughts on structural components of Chinese medicine combined with bioinformatics[J]. China Journal of Chinese Materia Medica,2015,40(22):4514−4519. [10] DENG Y L, REN H M, YE X W, et al. Integrated phytochemical analysis based on UPLC-Q-TOF-MS/MS, network pharmacology, and experiment verification to explore the potential mechanism of Platycodon grandiflorum for chronic bronchitis[J]. Frontiers in Pharmacology,2020,11:564131. doi: 10.3389/fphar.2020.564131
[11] XIE Y T, LIU Y, ZHENG P, et al. Based on UPLC-Q-TOF-MS/MS, systematic network pharmacology, and molecular docking to explore the potential mechanism of Fructus aurantii for major depression disorder[J]. Evidence-based Complementary and Alternative Medicine,2021,2021:6486287.
[12] AN L, YUAN Y L, MA J W, et al. NMR-based metabolomics approach to investigate the distribution characteristics of metabolites in Dioscorea opposita Thunb. cv. Tiegun[J]. Food Chemistry,2019,298:125063. doi: 10.1016/j.foodchem.2019.125063
[13] SUNGHWAN K, THIESSEN P A, BOLTON E E, et al. PubChem substance and compound databases[J]. Nucleic Acids Research,2016(D1):D1202−D1213.
[14] WISHART D S, GUO A C, OLER E, et al. HMDB 5.0: The human metabolome database for 2022[J]. Nucleic Acids Research,2021,50(D1):D622−D631.
[15] ZENG X, ZHANG P, WANG Y L, et al. CMAUP: A database of collective molecular activities of useful plants[J]. Nuclc Acids Research,2019,47(D1):D1118−D1127. doi: 10.1093/nar/gky965
[16] RU J L, LI P, WANG J N, et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines[J]. Journal of Cheminformatics,2014,16(6):13.
[17] DAINA A, MICHIELIN O, ZOETE V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules[J]. Nucleic Acids Res,2019,47(W1):W357−W364. doi: 10.1093/nar/gkz382
[18] WU T, HU E, XU S, et al. ClusterProfiler 4.0: A universal enrichment tool for interpreting omics data[J]. Innovation (N Y),2021,2(3):100141.
[19] HSU F, NONAKA G, NISHIOKA I. Tannins and related compounds. XXXIII. Isolation and characterization of procyanidins in Dioscorea cirrhosa Lour[J]. Chemical & Pharmaceutical Bulletin,2008,33(8):3293−3298.
[20] HAI L, TSIM K, CHOU G X, et al. Phenolic compounds from the rhizomes of Dioscorea bulbifera[J]. Chemistry & Biodiversity,2011,8(11):2110−2121.
[21] 曾涌, 罗建军, 何文生, 等. 薯蓣属植物化学成分及药理活性的研究进展[J]. 中国药房,2016,27(31):4454−4459. [ZENG Y, LUO J J, HE S W, et al. Research progress on chemical constituents and pharmacological activities of Dioscorea[J]. China Pharmacy,2016,27(31):4454−4459. doi: 10.6039/j.issn.1001-0408.2016.31.39 [22] ZENG X, LIU D, HUANG L. Metabolome profiling of eight Chinese yam (Dioscorea polystachya Turcz.) varieties reveals metabolite diversity and variety specific uses[J]. Life,2021,11:687. doi: 10.3390/life11070687
[23] 周丽. 山药叶中迷迭香酸和黄酮苷元的提取分离与抗氧化活性研究[D]. 北京: 中国矿业大学, 2016 ZHOU L. Study on separation, purification and antioxidant activities of rosmarinic acid and flavonoid aglycones from yam leaves[D]. Beijing: China University of Mining and Technology, 2016.
[24] 涂宝军, 马利华, 秦卫东, 等. 超声波辅助提取山药皮多酚工艺及酚类的鉴别研究[J]. 中国食品添加剂,2014(1):120−126. [TU B J, MA L H, QIN W D, et al. Studies on ultrasound-assisted extraction of yam peel polyphenol and its compositions[J]. China Food Additives,2014(1):120−126. doi: 10.3969/j.issn.1006-2513.2014.01.012 [25] WOO K W, KWON O W, KIM S Y, et al. Phenolic derivatives from the rhizomes of Dioscorea nipponica and their anti-neuroinflammatory and neuroprotective activities[J]. Journal of Ethnopharmacology,2014,155(2):1164−1170. doi: 10.1016/j.jep.2014.06.043
[26] SHUANG G A, YUE M B, YW B, et al. Effects of storage temperature on bisdemethoxycurcumin formation in fresh-cut yam (Dioscorea opposita)[J]. Journal of Food Composition and Analysis,2021,103:104106. doi: 10.1016/j.jfca.2021.104106
[27] YG, GUO X Y, LI X Y, et al. Organ- and age-specific differences of Dioscorea polystachya compounds measured by UPLC-QTOF/MS[J]. Chemistry & Biodiversity,2021,18(2):e2000856.
[28] 刘永录, 李自刚. 复合微生物制剂对怀山药连作障碍的修复机制研究[J]. 河南农业科学,2010(11):90−93. [LIU Y L, LI Z G. Study on the repair mechanis m of complex micro-biological additives to continuous cropping obstacle of Dioscorea opposita Thunb doi: 10.3969/j.issn.1004-3268.2010.11.024 J]. Journal of Henan Agricultural Sciences,2010(11):90−93. doi: 10.3969/j.issn.1004-3268.2010.11.024
[29] 黄佳颖, 杨悦, 李玉荣, 等. 芹菜素的研究进展[J]. 江苏科技信息,2015(4):57−58. [HUANG J Y, YANG Y, LI Y R, et al. Research progress of apigenin[J]. Jiangsu Science & Technology Information,2015(4):57−58. doi: 10.3969/j.issn.1004-7530.2015.04.023 [30] 马纳, 李亚静, 范吉平. 金合欢素药理研究进展[J]. 中国现代应用药学,2018,35(10):1591−1595. [MA N, LI Y J, FAN J P. Research progress on pharmacological action of acacetin[J]. Chinese Journal of Modern Applied Pharmacy,2018,35(10):1591−1595. doi: 10.13748/j.cnki.issn1007-7693.2018.10.035 [31] 胡昆, 杨泽华, 刘显华, 等. 甘草素的合成及其抗癌活性[J]. 合成化学,2010,18(4):513−516. [HU K, YANG Z H, LIU X H, et al. Synthesis and antitumor activities of liquiritigenin[J]. Chinese Journal of Synthetic Chemistry,2010,18(4):513−516. doi: 10.3969/j.issn.1005-1511.2010.04.031 [32] JIN F, CHEN X, YAN H, et al. Bisdemethoxycurcumin attenuates cisplatin-induced renal injury through anti-apoptosis, anti-oxidant and anti-inflammatory[J]. European Journal of Pharmacology,2020,874:173026. doi: 10.1016/j.ejphar.2020.173026
[33] LU Y, JIN M, PARK S J, et al. Batatasin I, a naturally occurring phenanthrene derivative, isolated from tuberous roots of Dioscorea batatas suppresses eicosanoids generation and degranulation in bone marrow derived-mast cells[J]. Biological & Pharmaceutical Bulletin,2011,34(7):1021−1025.





 下载:
下载:
 下载:
下载:
