Determination and Analysis of 16 Mycotoxins in Medicinal and Edible Traditional Chinese Medicine
-
摘要: 目的:建立同位素标记-超高效液相色谱-串联质谱法(UPLC-MS/MS)测定药食同源中药材中16种真菌毒素的分析方法,并利用该方法对市售的483份药食同源样品进行检测分析。方法:样品用乙腈-水(50/50,V/V)提取,MycoSpinTM 400多毒素净化柱净化,经Acquity UPLC BEH C18色谱柱(100 mm×2.1 mm,1.7 μm)分离,质谱采用电喷雾电离源(ESI),多反应监测模式(MRM)进行检测分析,同位素内标法进行定量。结果:16种真菌毒素标准曲线线性关系良好(R>0.998),方法的检出限在0.1~4.0 μg/kg之间,高、中、低3个不同浓度加标回收率为83.4%~102.3%,相对标准偏差(RSD,n=6)为2.08%~13.6%。483份样品中,共检出10种真菌毒素,其余6种真菌毒素均未检出,检出率最高的毒素化合物为玉米赤霉烯酮(ZEN),阳性样品中平均含量为71.2 μg/kg,并且有3.11%样品超过国家食品安全标准规定的参考限量。结论:该方法采用同位素稀释,多毒素净化柱对样品进行净化,降低了药食同源样品中基质干扰,方法准确、快速,满足测定方法的要求,可用于大批量样品中真菌毒素的多残留检测分析。
-
关键词:
- 药食同源 /
- 中药材 /
- 真菌毒素 /
- 超高效液相色谱-串联质谱法(UPLC-MS/MS) /
- 同位素标记
Abstract: Objective: An analytical method was established for the determination of 16 mycotoxins in traditional Chinese medicines (TCM) by isotope labeling-ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS), and 483 medicinal and edible homologous samples from the markets were detected using this method. Method: The samples were extracted with acetonitrile-water (50/50, V/V), and then purified by the MycoSpinTM 400 multifunction clean-up columns. Then the samples were detected and confirmed by UPLC-MS/MS, and quantified by isotope labeled internal standards. Rapid separation of 16 mycotoxins was successfully achieved on an Acquity UPLC BEH C18 column(100 mm×2.1 mm, 1.7 μm)with gradient elution. Simultaneous acquisition was performed in multiple reaction monitoring (MRM) mode with electrospray ionization (ESI) source operated in both positive and negative ionization modes. Result: The established method provided good linearities for the 16 mycotoxins within their respective linear ranges with high correlation coefficients (R>0.998), and the detection limits of this method were 0.1~4.0 μg/kg. The average recoveries of the 16 mycotoxins ranged from 83.4% to 102.3% at the three spiked levels, and the relative standard deviations (RSD, n=6) were in the range of 2.08%~13.6%. In the detection of 483 actual samples, 10 mycotoxins were detected, and the other 6 mycotoxins were not detected. The toxin compound with the highest detection rate was zearalenone (ZEN), with an average content of positive samples 71.2 μg/kg, and 3.11% of the samples exceeded the reference limit specified in the national food safety standard. Conclusion: This method used isotope dilution and multifunction clean-up columns to purify the samples, which reduced the matrix interference in the medicinal and edible homologous samples. The detection limits could meet the requirement of methods. The method was accurate and rapid, and could be used for the detection and analysis of multi residues of mycotoxins in a large number of samples. -
真菌毒素是一类由产毒真菌在一定环境条件下产生的具有毒性作用的次级代谢产物,目前已知的真菌毒素有300余种[1-2],其中常见的易对人体产生危害的真菌毒素有:黄曲霉毒素、单端孢霉烯族类毒素、玉米赤霉烯酮、伏马毒素和赭曲霉毒素等。部分真菌毒素已被证实具有致癌、致畸、致突变作用,对人类健康造成很大威胁[3-5]。中药是我国医药学发展的民族瑰宝,随着国际社会对中医药价值的普遍认可,中药材的质量安全也备受关注。由于中药材在种植、采集、加工、储藏和运输过程中,诸多条件的变化如干燥不及时、贮存不当、加工制备处理不善等均易使其受到真菌污染,产生有毒的真菌毒素,不仅使其功效成分受到影响,进而可能影响中药的质量和疗效,甚至还会对用药者的健康造成一定的威胁[6-8]。尤其药食同源中药材由于其使用量及使用范围更加广泛,其质量安全备受重视。随着真菌毒素风险评估工作的深入,很多国家和地区开始对部分真菌毒素的含量进行了强制性规定,并制定了严格的限量标准[9-13]。
目前,国内外检测真菌毒素的方法主要有酶联免疫吸附(ELISA)法[14-16]、薄层色谱(TLC)法[17]、高效液相色谱(LC)法[18-21]、气相色谱(GC)法[22]、气相色谱串联质谱(GC-MS)法[23]和液相色谱串联质谱(LC-MS/MS)法[24-29]等。由于真菌毒素的种类较多,且药食同源中药材基质比较复杂,同一样品可能污染多种真菌毒素,因此,建立同时测定多种毒素且准确可靠的方法非常必要,超高效液相色谱-串联质谱法检测真菌毒素的技术日渐成熟,应用广泛,能够满足检测需求。目前,大部分检测方法主要针对单类型目标物,而针对中药材中多种真菌毒素同时检测的方法SN/T 4604-2016适用范围窄,且多功能免疫亲和柱前处理操作复杂,定量方法采用外标法[30-33],不能满足同时检测复杂基质中多组分目标物的快速检测要求。
本研究拟采用新型的多真菌毒素快速净化柱,通过优化液相色谱-质谱条件,建立药食同源中药材中16种真菌毒素液相色谱-串联质谱检测方法,同位素内标法定量,以期建立能够校正基质效应,选择性好,灵敏度高,快捷、简便、高效,适用于大批量样品的快速测定方法。并拟采用该方法对采集的483份样品进行调查分析,为药食同源中药材中真菌毒素的安全风险评价监测提供技术支持。
1. 材料与方法
1.1 材料与仪器
药食同源中药材,采集市售药食同源样品共483份,按种类分:果实种子类药材340份,根及根茎类95份,动物类32份,藻菌类16份 均购自济南市大型药店;乙腈、甲醇、甲酸、氨水、乙酸 色谱纯,德国Merck公司;试验用水 Millipore纯水机制取超纯水;标准品:脱氧雪腐镰刀菌烯醇(DON,100.0 μg/mL)、雪腐镰刀菌烯醇(NIV,100.1 μg/mL)、黄曲霉毒素混合标准溶液(AFB1:2.02 μg/mL、AFB2:0.500 μg/mL、AFG1:2.02 μg/mL、AFG2:0.503 μg/mL)、HT-2毒素(HT-2,100.4 μg/mL)、T-2毒素(T-2,100.1 μg/mL)、伏马毒素B1(FB1,50.0 μg/mL)、伏马毒素B2(FB2,50.2 μg/mL)、伏马毒素B3(FB3,50.2 μg/mL)、玉米赤霉烯酮(ZEN,100.0 μg/mL)、赭曲霉毒素A(OTA,10.04 μg/mL)、3-乙酰基脱氧雪腐镰刀菌烯醇(3-ADON,100.0 μg/mL)、15-乙酰基脱氧雪腐镰刀菌烯醇(15-ADON,100.1 μg/mL)、Fus X毒素(FuX,100.3 μg/mL)、13C15-脱氧雪腐镰刀菌烯醇(13C15-DON,25.0 μg/mL)、13C15-雪腐镰刀菌烯醇(13C15-NIV,25.12 μg/mL)、13C17-黄曲霉毒素B1(13C17-AFB1,0.501 μg/mL)、13C17-黄曲霉毒素 B2(13C17-AFB2,0.502 μg/mL)、13C17-黄曲霉毒素G1(13C17-AFG1,0.501 μg/mL)、13C17-黄曲霉毒素-G2(13C17-AFG2,0.501 μg/mL)、13C24-T-2毒素(13C24-T-2,25.2 μg/mL)、13C22-HT-2毒素(13C22-HT-2,25.5 μg/mL)、13C34-伏马毒素B1(13C34-FB1,25.2 μg/mL)、13C34-伏马毒素B2(13C34-FB2,10.10 μg/mL)、13C34-伏马毒素B3(13C34-FB3,10.02 μg/mL)、13C18-玉米赤霉烯酮(13C18-ZEN,25.2 μg/mL)、13C20-赭曲霉毒素A(13C20-OTA,10.04 μg/mL)、13C17-3-乙酰基脱氧雪腐镰刀菌烯醇(13C17-3ADON,25.0 μg/mL)、13C17-15-乙酰基脱氧雪腐镰刀菌烯醇(13C17-15ADON,10.3 μg/mL) 均购自Romer Biopure公司。
Acquity UPLC I CLASS超高效液相色谱仪、XEVO-TQS串联质谱仪,配有电喷雾离子源(ESI) 美国Waters公司;VORTEX GENIE2涡旋混匀器 美国Scientific Industries公司;Millipore—Q超纯水系统 美国Millipore公司;CR21N型高速冷冻离心机 日本Hitachi公司;Multi Reax(EU)振荡混匀器 德国heidolph公司,MycoSpinTM 400多毒素净化柱 美国Romer Labs公司;ZM 200超离心粉碎研磨仪 德国Retsch公司;KQ5200E超声波清洗器 昆山市超声仪器有限公司。
1.2 实验方法
1.2.1 混合标准溶液的配制
准确移取标准溶液适量,用乙腈稀释并定容,配制成DON、3-ADON、15-ADON、NIV、T-2、HT-2、ZEN、FuX浓度为2 μg/mL,AFB1、AFG1为0.8 μg/mL,AFB2、AFG2为0.2 μg/mL,FB1、FB2、FB3、OTA为1.0 μg/mL的混合标准溶液,用于添加回收实验和制备标准系列溶液,−20 ℃避光条件下保存。
混合同位素内标溶液配制:分别准确移取各同位素标准溶液,用乙腈-水(10/90,V/V)溶液稀释并定容至25.0 mL,配制成13C15-DON、13C15-NIV、13C18-ZEN、13C17-3ADON、13C17-15ADON浓度为250 ng/mL,13C17-AFB1、13C17-AFB2、13C17-AFG1、13C17-AFG2为10.0 ng/mL,13C34-FB1、13C34-FB2、13C34-FB3、13C22-HT-2浓度为100 ng/mL,13C24-T-2为50 ng/mL,13C20-OTA浓度为20 ng/mL的内标混合溶液,−20 ℃避光条件下保存。
1.2.2 样品前处理
分别将样品用超离心粉碎研磨仪粉碎均匀(50目过筛),称取2.0 g(精确至0.01 g)粉碎后样品于50 mL离心管中,准确加入10 mL乙腈-水(50:50,V/V),涡旋混匀后,浸泡60 min,室温下超声提取30 min,10000 r/min室温下离心5 min,吸取1.0 mL上清液,加入50 μL乙酸,再加100 μL内标溶液,混合均匀,将混合液倒入MycoSpinTM 400多毒素净化柱中,涡旋2 min,使溶液与净化材料充分混匀,打开净化柱底部出液口,置于配套收集管中,收集液于10000 r/min离心1 min,取上清液过0.22 μm滤膜,上机检测。
1.2.3 色谱条件
Acquity UPLC BEH C18色谱柱(100 mm×2.1 mm, 1.7 μm,美国Waters公司);柱温40 ℃;流速0.3 mL/min;进样量为2 μL;ESI-模式:流动相A:0.2%氨水溶液,流动相B:乙腈,梯度洗脱程序:0 min,流动相B:2%;0~4.0 min,流动相B:2%~20%;4.0~5.5 min,流动相B:20%;5.5~5.6 min,流动相B:20%~100%;5.6~6.2 min,流动相B:100%;6.2~6.5 min,流动相B:100%~2%。ESI+模式:流动相A:0.1%甲酸水溶液,流动相B:乙腈+甲醇(50/50,V/V),梯度洗脱程序:0 min,流动相B:10%;0~1.0 min,流动相B:10%~30%;1.0~3.0 min,流动相B:30%~40%;3.0~5.0 min,流动相B:40%~70%;5.0~7.0 min,流动相B:70%;7.0~7.2 min,流动相B:70%~100%;7.2~7.7 min,流动相B:100%;7.7~8.0 min,流动相B:100%~10%。
1.2.4 质谱条件
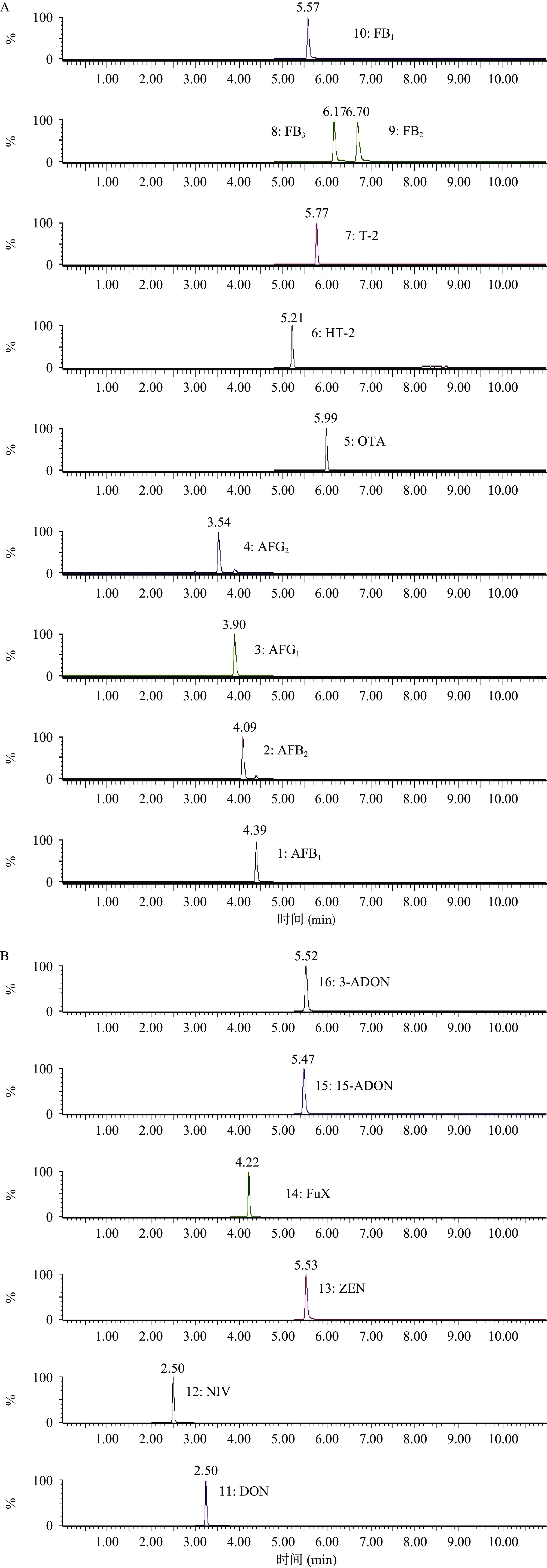
检测方式为多反应监测(MRM);ESI扫描方式为正、负离子扫描。离子源温度为150 ℃,去溶剂气温度:400 ℃;锥孔反吹气流速:150 L/h;去溶剂气流速:800 L/h;ESI+模式下毛细管电压为3.5 kV,ESI-模式下毛细管电压为2.0 kV;16种真菌毒素及内标的部分质谱参数见表1。
表 1 16种真菌毒素及对应的同位素内标的质谱优化参数Table 1. Optimized mass spectrometry parameters of 16 mycotoxins and their corresponding isotopic internal standards化合物 保留时间
(min)ESI模式 母离子
(m/z)子离子
(m/z)锥孔电压
(V)碰撞能量
(eV)AFB1 4.39 ESI+ 313 285*, 241 37 22, 34 AFB2 4.09 ESI+ 315 287*, 259 42 24, 28 AFG1 3.90 ESI+ 329 243*, 200 36 26, 40 AFG2 3.54 ESI+ 331 313*, 245 36 26, 28 FB1 5.57 ESI+ 722 334*, 352 42 38, 38 FB2 6.70 ESI+ 706 336*, 318 40 36, 34 FB3 6.17 ESI+ 706 336*, 318 40 36, 34 HT-2 5.21 ESI+ 425 263*, 245 15 12, 12 T-2 5.77 ESI+ 484 305*, 185 15 12, 20 OTA 5.99 ESI+ 404 239*, 221 44 22, 34 DON 3.24 ESI- 295 265*, 138 24 12, 12 NIV 2.50 ESI- 311 281*, 191 15 10, 20 ZEN 5.53 ESI- 317 175*, 131 16 22, 28 FuX 4.22 ESI- 353 263*, 187 18 10, 22 3-ADON 5.52 ESI- 337 307*, 173 17 10, 10 15-ADON 5.47 ESI- 337 150*, 219 36 22, 10 13C17-AFB1 4.39 ESI+ 330 301 37 20 13C17-AFB2 4.09 ESI+ 332 303 42 24 13C17-AFG1 3.90 ESI+ 346 257 36 28 13C17-AFG2 3.54 ESI+ 348 330 36 24 13C34-FB1 5.57 ESI+ 756 356 42 38 13C34-FB2 6.70 ESI+ 740 358 40 32 13C34-FB3 6.17 ESI+ 740 358 40 32 13C22-HT-2 5.21 ESI+ 447 278 15 13 13C24-T-2 5.77 ESI+ 508 322 15 15 13C20-OTA 5.99 ESI+ 424 250 50 22 13C15-NIV 2.50 ESI- 326 295 15 12 13C15-DON 3.24 ESI- 310 279 16 10 13C17-3ADON 5.52 ESI- 354 323 17 8 13C17-15ADON 5.47 ESI- 354 158 18 22 13C18-ZEN 5.53 ESI- 335 185 16 24 注:*为定量离子。 1.2.5 方法学考察
1.2.5.1 线性关系、检出限与定量限的考察
准确吸取16种真菌毒素混合标准溶液适量,用乙腈-水(10/90,V/V)溶液配制成系列梯度混合标液(AFB1、AFG1为0.32、0.8、2、4、8、20、40、80 ng/mL;AFB2、AFG2为0.08、0.2、0.5、1.0、2.0、5.0、10、20 ng/mL;FB1、FB2、FB3、OTA为0.4、1.0、2.5、5.0、10、25、50、100 ng/mL;其余毒素浓度为0.8、2.0、5.0、10、20、50、100、200 ng/mL),取1.0 mL混合标液,加入50 μL乙酸,100 μL混合同位素内标溶液,涡旋混匀后过0.22 μm滤膜,上机检测。以不同浓度的各目标物的峰面积与其内标峰面积比值对应相应的质量浓度进行线性回归,绘制标准曲线。按照三倍信噪比(3 S/N)计算检出限,10倍信噪比(10 S/N)为定量限。
1.2.5.2 加标回收率、精密度试验
选择杏仁、山药、鸡内金、茯苓4种基质空白样品,分别加入低、中、高三个水平的混合标准溶液,每个加标水平进行6次平行测定,按照1.2.2方法进行前处理,液相色谱质谱联用仪分析,根据测定结果计算三个加标水平下的回收率及其相对标准偏差,以验证该方法的准确性。
1.3 数据处理
采用内标法进行定量,应用Waters Masslynx仪器配置工作站系统进行数据采集处理,并使用Excel 2010进行数据分析。
2. 结果与分析
2.1 质谱条件的优化
将16种真菌毒素以及15种同位素内标分别用乙腈-水(10/90,V/V)稀释配制成适宜浓度的标准溶液(50~100 ng/mL),用流动注射泵直接注入质谱仪,根据各目标化合物分子的化学电离性质,DON、3-ADON、15-ADON、NIV、ZEN、FuX采用负离子模式,AFB1、AFB2、AFG1、AFG2、FB1、FB2、FB3、HT-2、T-2、OTA采用正离子模式,对各化合物及其内标的母离子、子离子、锥孔电压、碰撞能量等质谱参数进行优化。优化后的质谱条件见表1。由于正负离子同时扫描会降低方法的灵敏度,本方法采用正、负离子检测模式分别对目标化合物进行测定。
2.2 色谱分离条件的优化
优化质谱条件后,进行液相色谱分离条件的优化。流动相的组成和配比会影响目标化合物的色谱行为,以及其离子化效率和检测灵敏度。经查阅文献以及根据各个化合物的分子结构特征,本实验比较了甲酸、氨水等溶剂的加入情况及乙腈、甲醇流动相系统的组成,结果发现,在负离子模式下,流动相有机相采用乙腈,水相中加入0.2%氨水后,有利于化合物的离子化,并可以得到较高的检测灵敏度和更好的分离度。故最终确定了乙腈-0.2%氨水作为流动相。在正离子模式下,流动相水相中加入0.1%甲酸,有机相采用甲醇-乙腈(1+1)条件下,各目标化合物分离度和灵敏度均更好。并通过优化梯度洗脱,各目标化合物达到较好的分离,色谱峰峰形良好。图1为16种真菌毒素标准溶液及其内标离子流图。
2.3 样品净化条件的优化
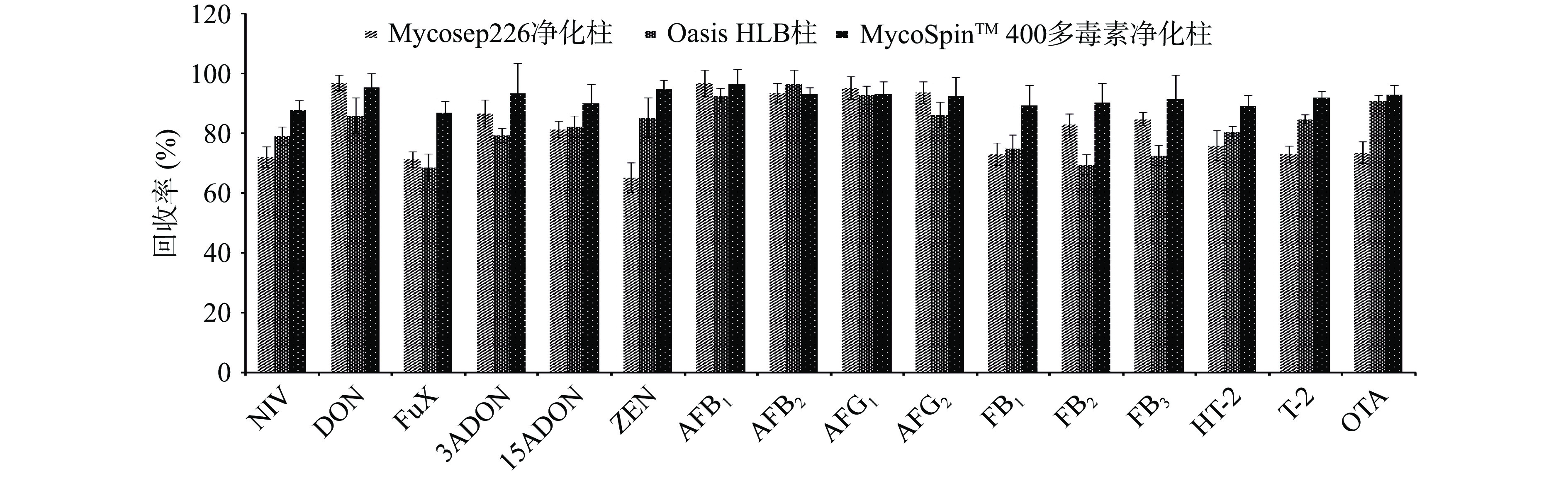
药食同源中药材基质比较复杂,为了能够更好的去除干扰物,降低基质效应,本实验对样品的净化条件进行了优化,由于多毒素免疫亲和柱前处理复杂,耗时较长,可测的毒素种类不能覆盖待测的16种毒素,本实验以鸡内金提取液配制基质标准溶液只针对Mycosep226净化柱、Oasis HLB柱和MycoSpinTM 400多毒素净化柱进行了比较,结果如图2所示。Mycosep226净化柱和Oasis HLB柱净化效果不理想,对ZEN、伏马毒素等的回收率较低。因此本实验采用MycoSpinTM 400多毒素净化柱对样品进行净化,可以降低药食同源中药材复杂基质对毒素检测的干扰,达到净化效果。
2.4 定量方法的确定
应用UPLC-MS/MS对复杂基质样品中目标化合物进行分析时,为了校正样品净化过程和离子化过程的损失以及减少基质效应,保证定量结果的准确可靠,通常采用基质匹配溶液加标法或空白溶剂内标法进行定量测定。由于药食同源中药材基质多种多样,而本文拟建立适用性更高的多种毒素同时测定的方法,因此,选择应用空白溶剂内标法进行定量检测。在内标化合物的选择上主要考虑目标化合物的结构、性质尽可能相似,除FuX外,本研究使用各个化合物的稳定性同位素[13C]作为指示内标,由于FuX难以找到同位素内标,因此,使用性质接近的13C15-DON作为其内标物进行检测。
2.5 工作曲线和检出限、定量限
本方法所确定的实验条件下,用乙腈-水(10/90,V/V)溶液配制系列浓度的真菌毒素标准溶液并分别进行测定,以相应真菌毒素的浓度为横坐标(X),目标物的峰面积与同位素内标的峰面积之比为纵坐标绘制工作曲线。结果表明,在一定的浓度范围内,各化合物呈良好的线性关系(R>0.998),线性回归方程、相关系数见表2,并以3倍信噪比(3 S/N)和10倍信噪比(10 S/N)计算检出限和定量限,各真菌毒素的检出限在0.1~4.0 μg/kg之间,定量限为0.3~10.0 μg/kg。
表 2 16种真菌毒素的线性方程、相关系数、线性范围、检出限和定量限Table 2. Linear equations, correlation coefficients, linear ranges, detection limits and quantification limits of 16 mycotoxins化合物 回归方程 相关系数 线性范围
(ng/mL)检出限
(μg/kg)定量限
(μg/kg)NIV Y=0.87017X+0.05828 0.9996 0.8~200 1.0 3.0 DON Y=0.72452X+0.09469 0.9993 0.8~200 1.0 3.0 FuX Y=0.52496X+0.001116 0.9987 0.8~200 1.0 3.0 3-ADON Y=0.90041X+0.09749 0.9999 0.8~200 2.0 5.0 15-ADON Y=0.41902X−0.16736 0.9980 0.8~200 2.0 5.0 ZEN Y=0.83662X+0.17959 0.9999 0.8~200 1.0 3.0 AFB1 Y=0.55309X−0.04670 0.9993 0.32~80 0.1 0.3 AFB2 Y=0.70547X+0.001443 0.9998 0.08~20 0.1 0.3 AFG1 Y=0.57448X−0.001124 0.9994 0.32~80 0.1 0.3 AFG2 Y=0.54956X−0.002338 0.9995 0.08~20 0.1 0.3 HT-2 Y=0.74764X−0.21247 0.9991 0.8~200 4.0 10.0 T-2 Y=1.41774X+0.27895 0.9994 0.8~200 1.0 3.0 OTA Y=0.64432X+0.001528 0.9990 0.4~100 0.5 1.5 FB1 Y=0.53930X+0.55478 0.9988 0.4~100 4.0 10.0 FB2 Y=0.39603X+0.53130 0.9991 0.4~100 4.0 10.0 FB3 Y=0.28763X+0.43231 0.9993 0.4~100 4.0 10.0 2.6 精密度与回收率试验
分别称取一定质量的不同基质的空白样品杏仁(果实种子类)、山药(根及根茎类)、鸡内金(动物类)、茯苓(藻菌类),添加3个浓度水平真菌毒素混合标准溶液,按实验方法进行处理测定,每个添加水平平行测定6份,考察方法的回收率与重现性,结果见表3。不同基质样品16种真菌毒素的加标回收率范围为83.4%~102.3%,相对标准偏差(RSD,n=6)为2.08%~13.6%。说明方法回收率与精密度良好。
表 3 精密度及回收率试验结果(n=6)Table 3. Results of test for precision and recovery(n=6)化合物 加入量
(μg/L)杏仁 山药 鸡内金 茯苓 回收率(%) RSD(%) 回收率(%) RSD(%) 回收率(%) RSD(%) 回收率(%) RSD(%) NIV 10 94.4 10.2 92.4 7.02 93.2 10.4 94.3 9.23 50 96.6 6.45 93.9 10.0 96.4 6.42 94.8 6.35 200 97.1 6.61 93.9 8.24 97.5 3.44 93.4 7.33 DON 10 102.3 9.82 93.6 5.52 96.6 4.78 93.5 9.48 50 96.6 6.69 99.9 4.92 101.0 2.30 96.6 6.91 200 97.0 5.19 101.1 2.89 99.2 4.32 93.3 8.16 FuX 10 96.2 13.6 83.7 4.95 101.2 12.3 92.3 8.98 50 88.3 10.1 92.8 9.34 98.2 3.53 101.0 2.74 200 95.7 6.23 87.6 7.10 88.1 6.72 90.5 10.8 3ADON 10 96.7 11.7 94.5 6.24 95.8 10.2 92.2 9.44 50 96.5 6.23 100.9 2.08 97.5 4.70 99.1 3.20 200 96.1 5.14 92.0 11.4 94.8 7.99 98.3 6.36 15ADON 10 95.8 13.4 83.4 6.89 102.0 8.45 90.9 9.70 50 95.2 5.74 93.0 9.74 94.2 9.94 94.2 9.64 200 98.8 6.12 96.0 9.88 93.6 8.75 93.0 11.2 ZEN 10 92.2 10.1 95.4 9.63 97.8 5.16 92.2 9.12 50 101.2 5.05 97.7 4.38 91.8 9.55 98.6 2.68 200 96.6 3.75 95.8 5.47 97.5 3.16 93.3 10.2 AFB1 4 94.8 9.29 95.5 6.98 95.8 5.53 95.7 7.86 20 96.0 8.39 97.0 5.09 92.9 6.54 94.7 6.60 80 99.3 2.40 96.1 6.30 96.7 5.69 94.1 7.79 AFB2 1 98.0 12.3 93.7 8.72 93.5 8.02 93.0 9.55 5 98.6 4.66 96.6 6.38 97.7 3.37 94.6 6.48 20 93.8 10.8 94.6 9.74 94.8 9.11 91.7 9.61 AFG1 4 94.2 13.5 95.8 6.83 93.5 8.67 95.9 5.64 20 94.6 10.5 94.4 8.34 92.7 8.50 94.8 6.37 80 95.3 8.10 97.3 4.49 97.4 4.15 95.0 7.99 AFG2 1 90.3 11.4 91.5 9.52 92.8 7.93 92.8 8.76 5 96.8 6.45 95.8 5.92 97.2 3.27 94.6 7.42 20 94.8 8.71 94.9 8.06 97.8 7.24 94.8 8.25 FB1 5 95.3 9.64 94.2 7.00 98.4 3.16 94.4 6.96 25 91.9 10.8 95.3 5.65 93.5 10.3 94.5 7.31 100 92.2 8.03 96.6 7.93 94.7 7.26 94.9 8.94 FB2 5 92.8 10.9 94.4 6.89 93.2 7.51 91.9 7.08 25 94.2 10.2 96.8 8.66 93.9 11.9 95.8 7.34 100 92.2 11.0 93.8 10.7 90.1 12.4 92.2 10.3 FB3 5 93.2 9.06 92.7 7.95 93.9 6.53 98.2 3.51 25 93.1 9.44 95.6 7.19 89.6 8.22 96.4 7.19 100 94.5 7.38 95.8 7.06 90.9 10.0 100.5 3.75 HT-2 10 93.6 8.06 96.6 6.51 94.4 9.42 93.8 8.44 50 98.5 4.27 101.5 2.16 93.8 8.42 92.2 8.94 200 99.1 3.85 101.4 3.31 89.4 9.14 93.9 8.79 T-2 10 93.1 7.31 95.4 7.04 92.6 10.5 93.8 11.6 50 97.2 6.44 102.0 4.26 91.2 9.04 95.9 6.33 200 95.9 9.84 97.8 4.65 92.4 5.85 93.5 4.87 OTA 5 95.3 12.4 100.5 2.66 92.8 7.81 95.4 7.48 25 99.7 4.31 95.6 8.70 96.7 7.41 95.1 6.74 100 98.6 8.92 92.5 9.05 94.3 8.15 98.4 9.59 2.7 实际样品的检测
利用已建立的方法,采集市售药食同源样品483份,包括果实种子类药材340份,根及根茎类药材95份,动物类药材32份,藻菌类药材16份,其中,阳性样品中检出各种真菌毒素的平均值及检出率见表4,共检出阳性样品77份,检出率15.9%,其中,果实种子类药材检出率为16.8%,动物类药材检出率34.4%,根及根茎类检出率9.47%。检出毒素种类最多的是薏苡仁,其中,一份薏苡仁样品检出6种毒素,两份检出5种毒素;其次是鸡内金,有六份样品检出4种毒素。毒素检出率较高的药材分别是枳椇子(4/4,检出批次/总批次)、薏苡仁(10/11)、鸡内金(11/13)、大麦芽(7/14)和桃仁(4/9),其中,在枳椇子和鸡内金的阳性样品中均检出玉米赤霉烯酮(ZEN)。
表 4 真菌毒素平均值及检出率Table 4. Mean values and detection rates of mycotoxins指标 DON ZEN AFB1 AFB2 AFG1 OTA FB1 FB2 FB3 NIV 平均值(μg/kg) 52.9 71.2 9.9 3.9 0.9 4.5 189.5 45.5 21.5 109.1 检出率(%) 4.8 7.9 2.1 0.4 0.2 0.4 5.8 5.6 3.9 2.7 注:平均值:阳性样品中毒素的平均值。 3. 结论
本文建立了同位素标记-超高效液相色谱/质谱法检测药食同源中药材中16种真菌毒素的检测方法。通过优化样品前处理方法、色谱条件和质谱条件,目标化合物分离度及线性关系良好,而且方法操作简单,准确、可靠,适用于药食同源中药材中多真菌毒素的检测,并利用此方法对市售483份药材进行了检测,为药食同源中药材中真菌毒素污染状况调查提供了数据支持。但是,由于具体到每种药材的样品数量较少,覆盖面欠缺,为了研究具体药材的污染情况,下一步需要针对具体的药材加大采样量,得到更加全面、有代表性的数据结果。
-
表 1 16种真菌毒素及对应的同位素内标的质谱优化参数
Table 1 Optimized mass spectrometry parameters of 16 mycotoxins and their corresponding isotopic internal standards
化合物 保留时间
(min)ESI模式 母离子
(m/z)子离子
(m/z)锥孔电压
(V)碰撞能量
(eV)AFB1 4.39 ESI+ 313 285*, 241 37 22, 34 AFB2 4.09 ESI+ 315 287*, 259 42 24, 28 AFG1 3.90 ESI+ 329 243*, 200 36 26, 40 AFG2 3.54 ESI+ 331 313*, 245 36 26, 28 FB1 5.57 ESI+ 722 334*, 352 42 38, 38 FB2 6.70 ESI+ 706 336*, 318 40 36, 34 FB3 6.17 ESI+ 706 336*, 318 40 36, 34 HT-2 5.21 ESI+ 425 263*, 245 15 12, 12 T-2 5.77 ESI+ 484 305*, 185 15 12, 20 OTA 5.99 ESI+ 404 239*, 221 44 22, 34 DON 3.24 ESI- 295 265*, 138 24 12, 12 NIV 2.50 ESI- 311 281*, 191 15 10, 20 ZEN 5.53 ESI- 317 175*, 131 16 22, 28 FuX 4.22 ESI- 353 263*, 187 18 10, 22 3-ADON 5.52 ESI- 337 307*, 173 17 10, 10 15-ADON 5.47 ESI- 337 150*, 219 36 22, 10 13C17-AFB1 4.39 ESI+ 330 301 37 20 13C17-AFB2 4.09 ESI+ 332 303 42 24 13C17-AFG1 3.90 ESI+ 346 257 36 28 13C17-AFG2 3.54 ESI+ 348 330 36 24 13C34-FB1 5.57 ESI+ 756 356 42 38 13C34-FB2 6.70 ESI+ 740 358 40 32 13C34-FB3 6.17 ESI+ 740 358 40 32 13C22-HT-2 5.21 ESI+ 447 278 15 13 13C24-T-2 5.77 ESI+ 508 322 15 15 13C20-OTA 5.99 ESI+ 424 250 50 22 13C15-NIV 2.50 ESI- 326 295 15 12 13C15-DON 3.24 ESI- 310 279 16 10 13C17-3ADON 5.52 ESI- 354 323 17 8 13C17-15ADON 5.47 ESI- 354 158 18 22 13C18-ZEN 5.53 ESI- 335 185 16 24 注:*为定量离子。 表 2 16种真菌毒素的线性方程、相关系数、线性范围、检出限和定量限
Table 2 Linear equations, correlation coefficients, linear ranges, detection limits and quantification limits of 16 mycotoxins
化合物 回归方程 相关系数 线性范围
(ng/mL)检出限
(μg/kg)定量限
(μg/kg)NIV Y=0.87017X+0.05828 0.9996 0.8~200 1.0 3.0 DON Y=0.72452X+0.09469 0.9993 0.8~200 1.0 3.0 FuX Y=0.52496X+0.001116 0.9987 0.8~200 1.0 3.0 3-ADON Y=0.90041X+0.09749 0.9999 0.8~200 2.0 5.0 15-ADON Y=0.41902X−0.16736 0.9980 0.8~200 2.0 5.0 ZEN Y=0.83662X+0.17959 0.9999 0.8~200 1.0 3.0 AFB1 Y=0.55309X−0.04670 0.9993 0.32~80 0.1 0.3 AFB2 Y=0.70547X+0.001443 0.9998 0.08~20 0.1 0.3 AFG1 Y=0.57448X−0.001124 0.9994 0.32~80 0.1 0.3 AFG2 Y=0.54956X−0.002338 0.9995 0.08~20 0.1 0.3 HT-2 Y=0.74764X−0.21247 0.9991 0.8~200 4.0 10.0 T-2 Y=1.41774X+0.27895 0.9994 0.8~200 1.0 3.0 OTA Y=0.64432X+0.001528 0.9990 0.4~100 0.5 1.5 FB1 Y=0.53930X+0.55478 0.9988 0.4~100 4.0 10.0 FB2 Y=0.39603X+0.53130 0.9991 0.4~100 4.0 10.0 FB3 Y=0.28763X+0.43231 0.9993 0.4~100 4.0 10.0 表 3 精密度及回收率试验结果(n=6)
Table 3 Results of test for precision and recovery(n=6)
化合物 加入量
(μg/L)杏仁 山药 鸡内金 茯苓 回收率(%) RSD(%) 回收率(%) RSD(%) 回收率(%) RSD(%) 回收率(%) RSD(%) NIV 10 94.4 10.2 92.4 7.02 93.2 10.4 94.3 9.23 50 96.6 6.45 93.9 10.0 96.4 6.42 94.8 6.35 200 97.1 6.61 93.9 8.24 97.5 3.44 93.4 7.33 DON 10 102.3 9.82 93.6 5.52 96.6 4.78 93.5 9.48 50 96.6 6.69 99.9 4.92 101.0 2.30 96.6 6.91 200 97.0 5.19 101.1 2.89 99.2 4.32 93.3 8.16 FuX 10 96.2 13.6 83.7 4.95 101.2 12.3 92.3 8.98 50 88.3 10.1 92.8 9.34 98.2 3.53 101.0 2.74 200 95.7 6.23 87.6 7.10 88.1 6.72 90.5 10.8 3ADON 10 96.7 11.7 94.5 6.24 95.8 10.2 92.2 9.44 50 96.5 6.23 100.9 2.08 97.5 4.70 99.1 3.20 200 96.1 5.14 92.0 11.4 94.8 7.99 98.3 6.36 15ADON 10 95.8 13.4 83.4 6.89 102.0 8.45 90.9 9.70 50 95.2 5.74 93.0 9.74 94.2 9.94 94.2 9.64 200 98.8 6.12 96.0 9.88 93.6 8.75 93.0 11.2 ZEN 10 92.2 10.1 95.4 9.63 97.8 5.16 92.2 9.12 50 101.2 5.05 97.7 4.38 91.8 9.55 98.6 2.68 200 96.6 3.75 95.8 5.47 97.5 3.16 93.3 10.2 AFB1 4 94.8 9.29 95.5 6.98 95.8 5.53 95.7 7.86 20 96.0 8.39 97.0 5.09 92.9 6.54 94.7 6.60 80 99.3 2.40 96.1 6.30 96.7 5.69 94.1 7.79 AFB2 1 98.0 12.3 93.7 8.72 93.5 8.02 93.0 9.55 5 98.6 4.66 96.6 6.38 97.7 3.37 94.6 6.48 20 93.8 10.8 94.6 9.74 94.8 9.11 91.7 9.61 AFG1 4 94.2 13.5 95.8 6.83 93.5 8.67 95.9 5.64 20 94.6 10.5 94.4 8.34 92.7 8.50 94.8 6.37 80 95.3 8.10 97.3 4.49 97.4 4.15 95.0 7.99 AFG2 1 90.3 11.4 91.5 9.52 92.8 7.93 92.8 8.76 5 96.8 6.45 95.8 5.92 97.2 3.27 94.6 7.42 20 94.8 8.71 94.9 8.06 97.8 7.24 94.8 8.25 FB1 5 95.3 9.64 94.2 7.00 98.4 3.16 94.4 6.96 25 91.9 10.8 95.3 5.65 93.5 10.3 94.5 7.31 100 92.2 8.03 96.6 7.93 94.7 7.26 94.9 8.94 FB2 5 92.8 10.9 94.4 6.89 93.2 7.51 91.9 7.08 25 94.2 10.2 96.8 8.66 93.9 11.9 95.8 7.34 100 92.2 11.0 93.8 10.7 90.1 12.4 92.2 10.3 FB3 5 93.2 9.06 92.7 7.95 93.9 6.53 98.2 3.51 25 93.1 9.44 95.6 7.19 89.6 8.22 96.4 7.19 100 94.5 7.38 95.8 7.06 90.9 10.0 100.5 3.75 HT-2 10 93.6 8.06 96.6 6.51 94.4 9.42 93.8 8.44 50 98.5 4.27 101.5 2.16 93.8 8.42 92.2 8.94 200 99.1 3.85 101.4 3.31 89.4 9.14 93.9 8.79 T-2 10 93.1 7.31 95.4 7.04 92.6 10.5 93.8 11.6 50 97.2 6.44 102.0 4.26 91.2 9.04 95.9 6.33 200 95.9 9.84 97.8 4.65 92.4 5.85 93.5 4.87 OTA 5 95.3 12.4 100.5 2.66 92.8 7.81 95.4 7.48 25 99.7 4.31 95.6 8.70 96.7 7.41 95.1 6.74 100 98.6 8.92 92.5 9.05 94.3 8.15 98.4 9.59 表 4 真菌毒素平均值及检出率
Table 4 Mean values and detection rates of mycotoxins
指标 DON ZEN AFB1 AFB2 AFG1 OTA FB1 FB2 FB3 NIV 平均值(μg/kg) 52.9 71.2 9.9 3.9 0.9 4.5 189.5 45.5 21.5 109.1 检出率(%) 4.8 7.9 2.1 0.4 0.2 0.4 5.8 5.6 3.9 2.7 注:平均值:阳性样品中毒素的平均值。 -
[1] 李雅静, 秦曙, 杨艳梅, 等. 中国谷物真菌毒素污染研究现状[J]. 中国粮油学报,2020,35(3):186−194. [LI Yajing, QIN Shu, YANG Yanmei, et al. Research status of mycotoxin contamination in grains in China[J]. Journal of the Chinese Cereals and Oils Association,2020,35(3):186−194. doi: 10.3969/j.issn.1003-0174.2020.03.030 [2] KIM D H, HONG S Y, KANG J W, et al. Simultaneous determination of multi-mycotoxins in cereal grains collected from South Korea by LC/MS/MS[J]. Toxins, 2017, 9(3): 106.
[3] COLLI L D, ELLIOTT C, FINNAN J, et al. Determination of 42 mycotoxins in oats using a mechanically assisted QuEChERS sample preparation and UHPLC-MS/MS detection[J]. Journal of Chromatography B,2020,1150:122187. doi: 10.1016/j.jchromb.2020.122187
[4] 王晓红, 豆金彦, 肖正国. 不同储藏条件下薏苡仁中真菌毒素含量变化的研究[J]. 西部中医药,2018,31(8):20−23. [WANG Xiaohong, DOU Jinyan, XIAO Zhengguo. Study on the content changes of fungal toxin in YiYiRen under different storage conditions[J]. Western Journal of Traditional Chinese Medicine,2018,31(8):20−23. doi: 10.3969/j.issn.1004-6852.2018.08.007 [5] MATUMBA L, NAMAUMBO S, NGOMA T, et al. Five keys to prevention and control of mycotoxins in grains: A proposal[J]. Global Food Security,2021,30:100562. doi: 10.1016/j.gfs.2021.100562
[6] 王少敏, 黄晓静, 毛丹, 等. QuEChERS-超高效液相色谱串联质谱法同时测定中药瓜蒌皮中22种真菌毒素[J]. 食品安全质量检测学报,2018,9(22):5843−5850. [WANG Shaomin, HUANG Xiaojing, MAO Dan, et al. Simultaneous determination of 22 kinds of mycotoxins in Trichosanthis pericarpium by ultra performance liquid chromatography-tandem mass spectrometry[J]. Journal of Food Safety and Quality,2018,9(22):5843−5850. doi: 10.3969/j.issn.2095-0381.2018.22.009 [7] FELIPE P T, RATCHANEEWAN K A, VERONIKA N, et al. Mycotoxins, phytoestrogens and other secondary metabolites in austrian pastures: Occurrences, contamination levels and implications of geo-climatic factors[J]. Toxins,2021,13(7):460. doi: 10.3390/toxins13070460
[8] 张鑫, 王福, 陈鸿平, 等. 中药材真菌及真菌毒素污染研究现状[J]. 世界科学技术-中医药现代化,2015,17(11):2381−2388. [ZHANG Xin, WANG Fu, CHEN Hongping, et al. Research progress on fungi and mycotoxin infection of Chinese medicinal materials[J]. World Science and Technology/Modernization of Traditional Chinese Medicine and Materia Medica,2015,17(11):2381−2388. doi: 10.11842/wst.2015.11.033 [9] FAO/WHO. Codex general standard for contaminants and toxins in food and feed: CODEX STAN 193-1995[EB/OL]. (2011-09-21) [2021-10-10]. http://down.foodmate.net/standard/sort/11/3329.html.
[10] Commission of the European Communities. Setting maximum levels for certain contaminants in foodstuffs: (EC) No 1881/2006 [EB/OL]. (2007-01-08) [2021-10-10]. http://law.foodmate.net/show-169747.html.
[11] Commission of the European Communities. A mending regulation (EC) No 1881/2006 as regards the maximum level of ochratoxin A in Capsicum spp. spices[EB/OL]. (2015-08-03) [2021-10-10]. http://law.foodmate.net/show-186931.html
[12] FDA. Guidance for industry: Action levels for poisonous or deleterious substances in human food and animal feed[EB/OL]. (2000-07-31) [2021-10-10]. https://www.regulations.gov/document/FDA-2020-D-1956-0001
[13] 中华人民共和国国家卫生和计划生育委员会, 国家食品药品监督管理总局. GB 2761-2017食品安全国家标准 食品中真菌毒素限量[S]. 北京: 中国标准出版社, 2017. National Health and Family Planning Commission of the People's Republic of China, China Food and Drug Administration. GB 2761-2017 National food safety standard: Limits of mycotoxins in foods[S]. Beijing: Standards Press of China, 2017.
[14] 王彤颖, 陈媛媛, 高璇, 等. 中药材中黄曲霉毒素B1免疫学快速检测方法研究[J]. 现代医药卫生,2017,33(2):177−179. [WANG Tongying, CHEN Yuanyuan, GAO Xuan, et al. Study on rapid immunological detection method for determining AFB1 in traditional Chinese medicines[J]. Journal of Modern Medicine & Health,2017,33(2):177−179. doi: 10.3969/j.issn.1009-5519.2017.02.005 [15] TANSAKUL N, JALA P, LAOPIEM S, et al. Co-occurrence of five Fusarium toxins in corn-dried distiller’s grains with solubles in Thailand and comparison of ELISA and LC-MS/MS for fumonisin analysis[J]. Mycotoxin Research,2013,29(4):255−260. doi: 10.1007/s12550-013-0173-z
[16] 柯颖, 徐昕怡, 洪小栩. ELISA法检测中药材及中药饮片真菌毒素的探讨[J]. 中国合理用药探索,2020,17(4):17−21. [KE Ying, XU Xinyi, HONG Xiaoxu. Discussion on the application of ELISA detection for mycotoxins in traditional Chinese medicine and Chinese herbal pieces[J]. Chinese Journal of Rational Drug Use,2020,17(4):17−21. [17] TEIXEIRA T R, HOELTZ M, EINLOFT T C, et al. Determination of ochratoxin A in wine from the southern region of Brazi by thin layer chromatography with a charge-coupled detector[J]. Food Additives & Contaminants: Part B,2011,4(4):289−293.
[18] ZHAO X, YUAN Y, ZHANG X, et al. Identification of ochratoxin A in Chinese spices using HPLC fluorescent detectors with immunoaffinity column cleanup[J]. Food Control,2014,46:332−337. doi: 10.1016/j.foodcont.2014.05.052
[19] 刘柱, 陈万勤, 沈潇水, 等. 多功能柱净化-柱后光化学衍生-高效液相色谱法同时检测玉米和花生中9种真菌毒素[J]. 分析科学学报,2014,30(2):168−172. [LIU Zhu, CHEN Wanqin, SHEN Xiaoshui, et al. Simultaneous determination of mycotoxins in corn and peanut samples by multifunctional column clean-up and high performance liquid chromatography coupled with post-column photochemical derivatization[J]. Journal of Analytical Science,2014,30(2):168−172. [20] ZHANG Yingyue, PEI Fei, FANG Yong, et al. Comparison of concentration and health risks of 9 Fusarium mycotoxins in commercial whole wheat flour and refined wheat flour by multi-IAC-HPLC[J]. Food Chemistry,2019,275:763−769. doi: 10.1016/j.foodchem.2018.09.127
[21] 邵亮亮, 应美蓉, 杜京霖, 等. 复合免疫亲和柱净化高效液相色谱法同时测定小麦中的4种真菌毒素[J]. 食品科技,2021,46(2):328−334. [SHAO Liangliang, YING Meirong, DU Jinglin, et al. Simultaneous determination of four mycotoxins in wheat by high performance liquid chromatography with combination immunoaffinity column purification[J]. Food Science and Technology,2021,46(2):328−334. [22] 张正炜, 成玮, 沈慧梅, 等. 小麦粉中脱氧雪腐镰刀菌烯醇(DON)毒素的气相色谱法检测及毒素污染去除分析[J]. 农药科学与管理,2018,39(4):44−50. [ZHANG Zhengwei, CHENG Wei, SHEN Huimei, et al. Determination of deoxynivalenol(DON) toxins in wheat flour by gas chromatography and analysis of toxin pollution removal[J]. Pesticide Science and Administration,2018,39(4):44−50. doi: 10.3969/j.issn.1002-5480.2018.04.014 [23] 林缨, 陈佳, 吴弼东, 等. 固相萃取-气相色谱-串联质谱法检测粮食作物中的T-2与HT-2毒素[J]. 军事医学,2013,37(5):381−384. [LIN Ying, CHEN Jia, WU Bidong, et al. Determination of T-2 and HT-2 toxins in cereal grains by solid phase extraction and gas chromatography-tandem mass spectrometry[J]. Military Medical Sciences,2013,37(5):381−384. [24] 范妙璇, 董娇娇, 王京辉, 等. QuEChERS-超高效液相-三重四极杆串联质谱测定白茅根中 16 种真菌毒素[J]. 中国中药杂志,2017,42(19):3770−3775. [FAN Miaoxuan, DONG Jiaojiao, WANG Jinghui, et al. Simultaneous determination of sixteen mycotoxins contaminants in cogon rootstalk by QuEChERS-UPLC-QqQ mass spectrometry[J]. China Journal of Chinese Materia Medica,2017,42(19):3770−3775. [25] 王少敏, 杜春晓, 刘贤贤, 等. QuEChERS-超高效液相色谱串联质谱法同时测定三七中26种真菌毒素[J]. 世界中医药,2019,14(4):798−804. [WANG Shaomin, DU Chunxiao, LIU Xianxian, et al. Simultaneous determination of 26 mycotoxins in Notoginseng Radix et Rhizoma by QuEChERS-ultra-high-performance liquid chromatography-tandem mass spectrometry[J]. World Chinese Medicine,2019,14(4):798−804. doi: 10.3969/j.issn.1673-7202.2019.04.003 [26] JIANG D, WEI D, Wang L, et al. Multiwalled carbon nanotube for one-step cleanup of 21 mycotoxins in corn and wheat prior to ultraperformance liquid chromatography-tandem mass spectrometry analysis[J]. Toxins,2018,10(10):409−425. doi: 10.3390/toxins10100409
[27] YAN Pianpian, LIU Zhezhe, LIU Shiqiao, et al. Natural occurrence of deoxynivalenol and its acetylated derivatives in Chinese maize and wheat collected in 2017[J]. Toxins,2020,12(3):200. doi: 10.3390/toxins12030200
[28] RAUSCH A K, BROCKMEYER R, SCHWERDTLE T. Development and validation of a QuEChERS-based liquid chromatography tandem mass spectrometry multi-method for the determination of 38 native and modified mycotoxins in cereals[J]. Agricultural and Food Chemistry,2020,68:4657−4669. doi: 10.1021/acs.jafc.9b07491
[29] 胡佳哲, 吴凤丹, 陈俏, 等. 同位素标记-高效液相色谱-串联质谱法测定中药材中8种真菌毒素[J]. 中国卫生检验杂志,2020,30(5):513−517. [HU Jiazhe, WU Fengdan, CHEN Qiao, et al. Determination of 8 mycotoxins in Chinese medical materials by isotope labeling-high performance liquid chromatography-tandem mass spectrometry[J]. Chinese Journal of Health Laboratory Technology,2020,30(5):513−517. [30] WU Yu, YE Jin, XUAN Zhihong, et al. Development and validation of a rapid and efficient method for simultaneous determination of mycotoxins in coix seed using one-step extraction and UHPLC-HRMS[J]. Food Additives & Contaminants: Part A,2021,38(1):148−159.
[31] 中华人民共和国国家质量监督检验检疫总局. SN/T 4604-2016 中华人民共和国出入境检验检疫行业标准 进出口中药材中真菌毒素的测定[S]. 北京: 中国标准出版社, 2017. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China. SN/T 4604-2016 Entry-exit inspection and quarantine industry standard of the People's Republic of China. Determination of mycotoxins in Chinese herbal medicine for import and export[S]. Beijing: Standards Press of China, 2017.
[32] 郑润生, 肖启衍, 邱薇, 等. 10种中药材和3种食品污染赭曲霉毒素A的LC-MS/MS检测研究[J]. 药物分析杂志,2015,35(2):289−294. [ZHENG Runsheng, XIAO Qiyan, QIU Wei, et al. Determination of contaminant ochratoxin A in 10 traditional Chinese medicines and 3 food samples by LC-MS/MS[J]. Chinese Journal of Pharmaceutical Analysis,2015,35(2):289−294. [33] 毛丹, 叶林链, 王少敏, 等. LC-MS/MS法同时测定中药肉豆蔻中12种真菌毒素[J]. 中国药师,2020,23(7):1311−1315. [MAO Dan, YE Linlian, WANG Shaomin, et al. Simultaneous determination of 12 mycotoxins in myristicae semen by LC-MS/MS[J]. China Pharmacist,2020,23(7):1311−1315. doi: 10.3969/j.issn.1008-049X.2020.07.015





 下载:
下载:
 下载:
下载:
